All published articles of this journal are available on ScienceDirect.
Gender Differences in Response to Immoral Behaviour in Living and Nonliving Beings: Electrophysiological Evidence
Abstract
Background:
Gender is a significant socio-biological determinant of psycho-moral development and contributes to eliciting greater P300 brain potential in the emotional cognition process associated with immoral behavioural patterns.
Objective:
To investigate the interaction between gender and the moral cognition process in different contexts of immoral behavior.
Methods:
Twenty-six participants (mean age 24 years old, 16 males) participated in the Event-Related Potential (ERP) session in the Neuroscience Laboratory. In a within-subject experimental design, males and females responded to the oddball task by viewing a random series of 200 trials consisting of different categories of images (i.e., immoral behaviour to living beings, immoral behaviour to nonliving beings, and neutral images). The electrical brain potential of the P300 component was captured using the international 10/20 system in several brain regions, i.e., frontoparietal, frontal, central, temporal, and occipital.
Results:
Females indicated greater P300 amplitude in the frontoparietal brain region than males. Both genders exhibited greater brain potential activation while responding to images of living beings than nonliving beings and neutral images.
Conclusion:
The frontoparietal region of the brain is the most significant area linked to the relationship between the processing of moral cognition and gender differences. In moral contextualising, females demonstrate greater emotional cognition than males. Immoral behaviour toward living beings generates a more humanistic sense than nonliving beings and neutral images, which are seen in both males and females. The discovery has important implications for understanding gender-associated moral cognition from a neuroscience perspective.
1. INTRODUCTION
Gender is a socio-biological factor that interacts with genetic and hormonal and plays an important role in human psychosocial development [1-5]. As gender is considered an important factor that influences how people behave and interact in society, it is a critical socio-biological determinant among young people in the conceptualization of moral behaviour[6-9]. Thus, controlling the gender factor in the analysis has been strongly recommended to deal with the complexity of health issues [10-13].
Whether and how gender differences manifest in moral judgement has recently been the focus of much research in the field of moral psychology [14-18]. Moral psychologists such as Capraro and Sippel [19] hypothesized that gender differences in moral dilemmas are driven by emotional salience rather than a violation of the practical imperative. In this case, they believe that women should behave differently than men in these situations. Jaffee and Hyde [20] conducted a meta-analysis to quantitatively review the research on gender differences in moral orientation. Despite the relatively small difference between males and females, there were differences in care orientation favouring females and differences in justice orientation favouring males.
The bio-behavioural model suggests that males and females have different capacities for empathy. This argument can be seen in Christov-Moore et al.'s review work [21], which stated that the neurobiological underpinnings of empathy reveal significant quantitative gender differences in the basic networks involved in affective and cognitive forms of empathy.
Moral violation refers to behaviour that violates social norms and is frequently judged by society [22-26]. It highlights three main schools of thought that address the idea of moral judgement - Piaget’s moral cognitive development model, the social intuitionist model, and the dual-processing theory [27-33]. The dual-processing theory and the social intuitionist model place special emphasis on moral emotions in people, which are a subset of basic human emotions like rage and disgust and have an impact on moral judgement [27, 28, 34].
A neuroscience approach was implemented in this study to determine whether there is a neurological basis for the study of moral intuition. Recent electroencephalogram (EEG) studies have demonstrated that different types of moral violation can affect how one perceives moral violation, with significant differences in brain wave amplitude observed (22, 35-36). According to the findings of Zhang et al. [36], moral elicitors produced amplitudes that were larger than those produced by core disgust and neutral images, indicating that moral emotions are distinct from core emotions in a neuroscientific sense. In a similar vein, research in the field of neuro-cognition has identified P300 as a neuro biomarker that can be used to explain the psychophysiological mechanisms underlying behavioural issues [37].
At least two critical points should be highlighted as gaps in this area of study. First, the interpretation of ‘gender influence’ is lacking in the above-mentioned studies, especially the evidence from the neuroscientific data (i.e. electrophysiological data). The use of event-related potential (ERP), for example, offers evidence of the brain-wave pattern (associated with the small alterations in the electroencephalogram) recorded on the scalp that is timed to the commencement of an event such as a sensory stimulus or a motor act. Electroencephalography offers a medium to comprehend neurobiological dysregulation, with the potential to explore neurotransmission. Thus, this ERP method is an appropriate approach, as compared to other neuroscience methods, due to its ability to explain the human psycho-behavioural dimension from the brain potential parameter, such as the P300 component. Second, there has been no previous study specifically considering the neural-morality properties associated with the different contexts of immoral behaviour. Therefore, it is hypothesized that emotional attention to the different contexts of immoral behaviour (i.e., living beings and non-living beings) is influenced by the gender factor.
2. METHODS
2.1. Research Design and Participants
Through the laboratory observation design that was held in Neuroscience Laboratory, the Event-Related Potential (ERP) technique was used to capture the brain activities while participants were stimulated with the visual stimulus. The target population was young Malaysian adults aged 18 to 35 years old. The participants in this study were 24 years old on average (SD±2.48), with 16 males and 10 females. Malay ethnicity made up the majority of participants (90%) and was followed by Chinese and Indian ethnicities (10%). In terms of education level, the majority of participants were pursuing their first degree in a local university located in Kota Bharu, Kelantan, Malaysia. All participants (N=26) were chosen based on the following inclusion criteria: no previous chronic medical illness or neuropsychiatric disease, right-handedness, and normal or corrected to normal vision.
The sample size is estimated by using G*Power 3.1.9.7 (UCLA Statistical Consulting) software. Based on G*Calculation, the calculated effect size f is 0.252 which is derived from the estimated effect size medium (partial eta squared = 0.06). The α error probability is 0.05, β error probability is set at 0.80, and the number of groups is 3. The correlation among the three groups is 0.5, along with non-sphericity correction ε of 1. The final power analysis showed that the estimated sample size is 28, and the actual power is 0.82. However, two participants were dropped in the last stage of analysis because of major brainwave errors.
2.2. Research Procedure
Study information was revealed to the participants through an advertisement that was disseminated on social media. Participants who were interested and met the inclusion criteria were chosen using the convenient sampling method, and they were given extensive information about the methods and procedure of the study. The Human Ethics Board approved the study's protocol (USM/JEPeM/20060297).
Participants completed a consent form and provided socio-demographic information before engaging in electroencephalography (EEG) recordings in the lab. They were asked to watch a set of neutral images and immoral behavior images that were shown on a computer screen while wearing the 128 HydroCel Geodesic Sensor Net that was placed on their heads. This sensor net was connected to a NetAmps 300 amplifier with a high input impedance to record their EEG response to the stimulus. The oddball paradigm was used, in which a deviant stimulus would occasionally interrupt the presentation of repeating stimuli in a sequence. Thus, the image presentation during the EEG recording followed the 1:1:3 ratio. This means that neutral images make up 70% of all images projected on the computer screen. Meanwhile, 30% of the images were target images (i.e., living and nonliving immoral behaviour). All images were randomly assigned to 200 trials with three times of repetition throughout the experiment. Participants’ behavioural reactions were also recorded. Pressing the ‘1’ key on the keyboard denotes responding to immoral behaviour toward living beings, and the ‘2’ key denotes immoral behaviour toward nonliving beings. No response was required for the neutral images (geometry images).
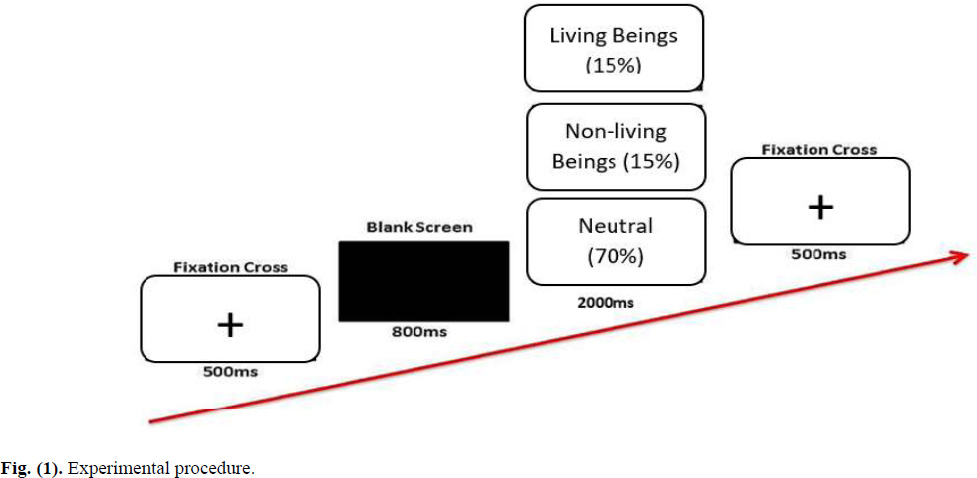
The fixation mark (+) was displayed in the middle of the monitor screen for 500 milliseconds to start the trial. Then, a black screen appeared for 800 milliseconds before a visual image appeared for 2000 milliseconds. The following trial began with the fixation cross, and so on. The entire session, from the beginning to the end of the brain recording, lasted about an hour. The experiment's procedure, as controlled by the E-prime programmer, is shown in Fig. (1). The study's protocol was approved by the Human Ethics Board.
2.3. Sociodemographic Information
Participants' age, gender, ethnicity, educational background, and current employment status were collected.
2.4. Visual Affective Stimulus
To conceptualize the neural underpinnings of moral categorization, two categories of images representing immoral behaviour were used as visual stimuli to elicit cognitive and emotional judgements. The first category is immoral behaviour toward living beings, e.g., killing animals, kicking people, and so on (10 images); the second is immoral behaviour toward nonliving beings, e.g. littering everywhere, disposal of human and animal waste/feces and so on (10 images). The geometrical images (30 images), which were designated as neutral images, were also shown, making up about 70% of the entire trial. All photographs (images) were obtained from the internet and are free of copyright.
Three experts in the field of moral behaviour evaluated the images' content validity. They were psychologists with extensive experience researching moral behaviour. Evaluators were instructed to use the following options to rate 20 images, of which ten images showed immoral behaviour toward living beings and ten images showed immoral behaviour toward nonliving beings. The options are as follows: 1= not relevant, 2 = somewhat relevant, 3 = quite relevant (given as X), and 4 = extremely relevant (given as X) [38]. The Individual Content Validity Index (I-CVI), which considers the X scores from Scores 3 and 4, obtained from each evaluator, illustrates the content validity of each image. The I-CVI was calculated using the formula below:
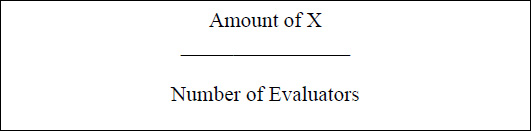
With an I-CVI score of one, all images showed strong content validity. When evaluating the validity of content, a score of one is considered acceptable. To remove technical bias, the images' brightness and size were standardised. Additionally, the copyright-free status of these images is guaranteed.
2.5. Data Extraction and Analysis
Brain potential data was obtained in five brain areas using the international 10/20 system of Ag/AgCl electrodes placed at scalp sites. These brain areas are: fronto-parietal (Fp1, Fp2), frontal (F3, F4, F7, F8, Fz), central (C3, C4, Cz), temporal (T3, T4, T5, T6), parietal (P3, P4, Pz) and occipital (O1, O2).
Prior to statistical analysis, ERP components were extracted using a variety of procedures. The raw EEG brain waves were filtered with the 0.3-30Hz noise reducer during the filtering process to remove noise from muscle movement or other electrical systems. With 45 milliseconds offset, segmentation was performed by locking it to 200 milliseconds before stimulus onset and 1000 milliseconds after stimulus onset. Following that, artefact detection was performed, which included removing eye movements and blinking (ocular artifacts). In a bad channel replacement procedure, bad channels that were bad in 20% of the recordings across all segments were interpolated using the signal provided by nearby good electrodes. To improve the signal-to-noise ratio, the data were then averaged separately. After baseline correction, the reported data wave was converted into a 10-10 EEG montage.
Due to the significant role that the ERP subcomponent of P300 plays in the processing of information and visual cognition, it was chosen for analysis. A 3 x 2 design was used in this experiment to determine the variations of P300 amplitude in various brain regions as induced by different immoral behaviours. The types of stimuli (neutral, immoral behaviour in living beings, and nonliving beings) were designed as within-subject factors. Meanwhile, gender was designed as the between-subject factor. In the case of spherical assumption violation, the degree of freedom (df) was adjusted to the new degree of freedom from the Epsilon Huynh-Feldt result. Other than that, reaction times that were extracted from E-Prime 2.0 software were also reported.
3. RESULTS
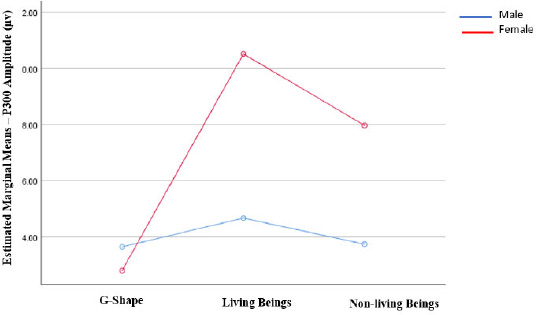
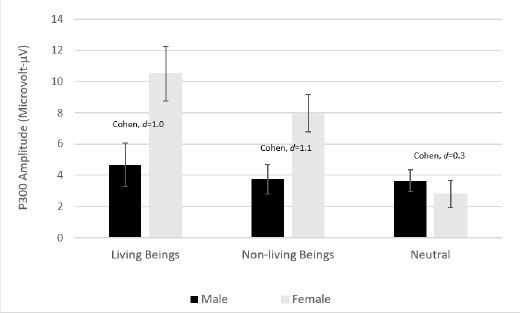
According to the results of the analysis of variance (ANOVA) for a two-way mixed design, which included gender as a between-subject effect and moral cognition P300 amplitude as a within-subject effect, there was a significant interaction between gender and electrical brain activity (as measured by P300 amplitude) in the frontoparietal area [F (2, 48) = 6.60; p0 .01]. Females revealed greater amplitude towards living beings and non-living beings stimuli than males, with an exemption to neutral stimuli. The interaction is depicted in Fig. (2). However, other brain regions did not show any interaction with gender, i.e., frontal [F (1.95, 46.79) = 2.98; ns], central [F (2, 48) = 0.47; ns], temporal [F (2, 48) = 1.40; ns], parietal [F (2, 48) = 0.38; ns] and occipital [F (2, 48) = 0.27; ns] (Table 1).
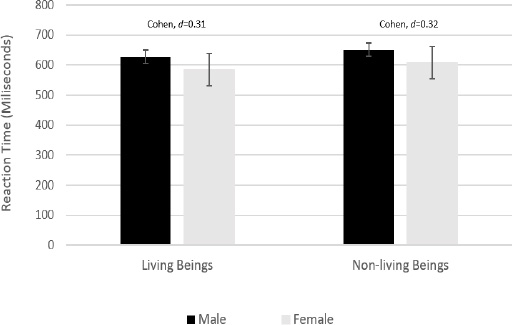
An effect size analysis was carried out for frontoparietal data to observe the difference in electrical brain activity (P300 amplitude) between males and females, specifically for each type of visual moral stimuli. It showed that Cohen’s-d is trivial for both living being and nonliving stimuli. Females exhibited greater amplitude means than males in both cases - Mean difference of 5.8 (SE 2.2) and 4.2 (SE 1.5), respectively. Meanwhile, in line with expectation, the effect size for neutral stimuli is small (Fig. 3).
| P300 Amplitude: Mean (SE)1 | F | df | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Living beings | Non-living beings | Neutral | ||||||
| M | F | M | F | M | F | ||||
| Frontoparietal | 4.67(1.38) | 10.52 (1.75) | 3.74 (0.94) | 7.97 (1.20) | 3.65 (0.69) | 2.81 (0.88) | 6.60 | 2.00 | 0.003* |
| Frontal | 2.29 (0.35) | 2.83 (0.44) | 1.90 (0.36) | 2.71 (0.46) | 1.93 (0.36) | 1.04 (0.46) | 2.98 | 1.95 | 0.062 |
| Central | 1.92 (0.29) | 2.23 (0.37) | 1.81 (0.39) | 1.42 (0.49) | 1.12 (0.26) | 1.29 (0.33) | 0.47 | 2.00 | 0.63 |
| Temporal | 5.70 (0.45) | 4.48 (0.57) | 4.06 (0.40) | 3.09 (0.50) | 1.16 (0.18) | 1.14 (0.23) | 1.40 | 2.00 | 0.26 |
| Parietal | 6.64 (0.78) | 6.97 (0.98) | 3.28 (0.56) | 3.74 (0.71) | 1.83 (0.27) | 1.33 (0.34) | 0.38 | 2.00 | 0.69 |
| Occipital | 8.66 (0.83) | 9.07 (1.05) | 6.61 (0.89) | 7.23 (1.12) | 2.20 (0.25) | 1.75 (0.32) | 0.27 | 2.00 | 0.77 |
Interestingly, behavioral data from participants' reactions to images of living beings (press button 1) and nonliving beings (press button 2) revealed a medium effect size between males and females, as reported by their reaction times in response to the stimuli (Fig. 4).
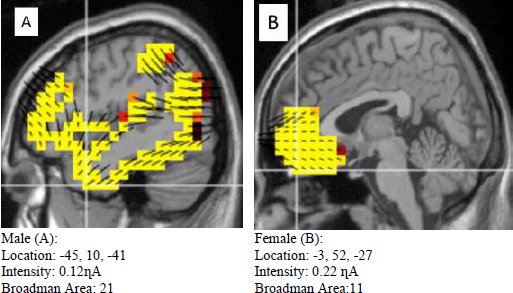
The difference in source localization between males and females in three conditions in response to living beings, non-living beings, and neutral images is depicted in Figures 5, 6 and 7. The living being category (Fig. 5) showed a clear distinction between males and females. The sagittal view revealed that males (A) showed more distributed activation than females (B). However, the intensity was one-fold higher in females (B) than in males (A). In terms of Broadman area, the values are considerably different, with females (B) being 11 and males (A) being 21.
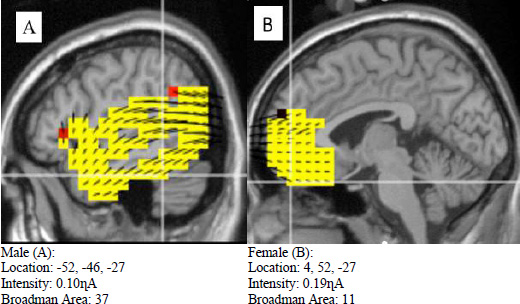
Similar results were observed in the nonliving category (Fig. 6), in which neural activation was more widespread in males (A) than females (B). However, it was shown that females (B) displayed greater intensity than males (A). Another great difference was that both males (A) and females (A) differed greatly in the Broadman area.
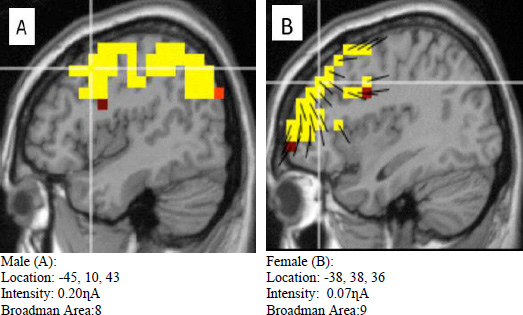
Meanwhile, in the neutral image category (Fig. 7), even though the intensity was relatively different between males (A) and females (B), the Broadman areas between these two genders were almost similar.
4. DISCUSSION
At least two important findings can be gleaned from this study. First, gender has had a significant impact on the processing of moral cognition, mainly in the frontoparietal area as indexed by P300 amplitude. The discovery of frontoparietal activation and its relationship to moral cognition processing requires the moral psychologist to pay close attention. Females showed significantly stronger moral cognition processing in the frontoparietal area, compared to males. Second, the frontoparietal region was more activated by living-being stimuli than non-living-being stimuli in both male and female genders.
Empirical research reported that females showed higher concern for a positive moral stream, such as care, fairness, and purity, in their moral judgments than males [39]. The discovery that females are less likely than males to respond in a utilitarian sense to moral dilemmas reflected females as more empathetic [40]. Utilitarianism is a moral philosophy that seeks to do the best for the greatest number of people while disregarding factors such as feelings and emotions, culture, and justice [41-43]. In a similar vein, Fumagalli et al. [44] reported that males provided significantly more useful responses to personal moral dilemmas, thus suggesting the difference between males and females in cognitive-emotional processes of personal dilemmas.
Evidence that females are more empathetic than males is becoming more widely accepted. In Derntl et al. [45], again, females were observed to rate themselves as more empathetic than males in the self-report questionnaires. Females demonstrated stronger neural activation in all empathy tasks in emotion-related regions, including the amygdala. Meanwhile, Rueckert et al. [46] found that females rated themselves as feeling happier and sadder than males, regardless of whether the event occurred to them, a friend, or an enemy. This suggests that differences in general emotional responsiveness may account for gender differences in empathetic responses.
We found that the frontoparietal brain area indicated greater activation when evoked by living being stimuli than nonliving being stimuli in both males and females. This discovery is significant because it adds to our understanding of earlier theories, such as the Moral Foundation Theory (MFT) - a thorough framework that looks at one's moral intuition and judgement [47-54]. As our data demonstrate, whether moral stimuli are living beings or nonliving beings, they have a significant impact on how people judge what is good and wrong. In other words, the capacity of moral stimuli might be what drives spontaneous action.
Neuro-cognition evidence in the neuroscience field has greatly contributed to explicating human morality [55-59]. We were not the first to realise the importance of the variation in moral stimuli that underlies moral cognition processing. According to a previous moral cognition model, different types of moral stimuli had distinct neurological foundations. For instance, Zhang et al. [36] discovered that core disgust stimuli had larger amplitudes of components N1, P2, and P3, whereas moral disgust stimuli had larger N2 amplitudes. Other than that, previous ERP research has shown that when subjects see someone else being hurt, different ERP components are involved. A longer electrocortical response lasting 200–300ms was seen over the centroparietal regions, which is followed by larger early ERPs over the frontocentral and frontal brain regions [60].
The discovery of the P300 neural component as a moral cognition marker, which we highlight in this study, has added to the existing literature. It is noted that the ERP modulations induced by emotional content are frequently characterized by increased activation of primary sites, including P300 [61]. Moral disgust stimuli, for example, increased P300 amplitude along with N200 and Late Positive Potential (LPP) amplitudes [36, 62]. Meanwhile, in multiple studies, the P300 component (as well as the P200 component) showed greater positive deflection in negative conditions than in neutral conditions. The P300, which frequently overlaps with LPP in earlier ERP studies, is frequently linked to attentional and memory processes [63-65].
The frontoparietal network has been recognised for its roles in human mental functioning, such as executive function and goal-oriented behaviour [66-69]. The role of the frontoparietal regions in abnormal emotional and cognition processing has also been highlighted in several scientific reports [70-73]. In the case of Social Anxiety Disorder (SAD), patients showed the ability to downregulate negative emotions using both reappraisal and acceptance and demonstrated effective recruitment of frontoparietal regulatory regions [74]. In different cases, the neural interface between negative emotion regulation and motivation for change in cocaine-addicted patients revealed emotional-related activation in the frontoparietal engaged during emotion regulation [75]. The important role of the frontoparietal as a set of regions involved in cognitive control of attention and emotion regulation was further highlighted by Kaiser et al. [76], who reported hypoconnectivity within the frontoparietal network in major depressive disorder. Major depressive disorder (MDD) has a connection with imbalanced communication among large-scale brain networks.
Nonetheless, this current discovery should consider the socio-demographic factors of the participants involved. Since they were university populations, this finding perhaps can not be generalized to a wider population. However, the novelty of the study, which looks specifically at the moral foundation of immoral behaviour (i.e., living and non-living beings of immoral behaviour), should be emphasized and highlighted as a research strength.
CONCLUSION
Gender and moral cognition interact primarily in the frontoparietal brain region. As indexed by the P300 amplitude, immoral behaviour toward living beings is associated with greater neural cognition processing in females than in males. This discovery contributes significantly to the field of moral psychology by improving the understanding of the moral foundation of neurophysiological evidence. This discovery contributes significantly to the field of moral psychology by providing a better understanding of moral foundations. This neuroscientific evidence could be an important referral for psychologists in mapping the gender-based patterns of moral empathy that link to brain function.
AUTHORS' CONTRIBUTIONS
Nasir Yusoff conceptualized, designed, and prepared the initial draft and framework and interpreted the data. Chin Chun Ming and Mohd Faizal Mohd Zulkifly reviewed the measurement adaptation, supervised the findings of the study, made corrections in English, and reviewed the manuscript.
All authors discussed the results and contributed to the final manuscript.
LIST OF ABBREVIATIONS
| ERP | = Event-Related Potential |
| EEG | = Electroencephalogram |
| I-CVI | = Individual Content Validity Index |
| F | = Freedom |
| NS | = Not Significant |
| MFT | = Moral Foundation Theory |
| N1 | = Negative 100 component |
| P2 | = Positive 200 component |
| P3 | = Positive 300 component |
| N2 | = Negative 200 component |
| SAD | = Social Anxiety Disorder |
| MDD | = Major depressive disorder |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the Universiti Sains Malaysia (USM/JEPeM/20060297).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Participants were explained the study’s objectives and purpose, and written consent to participate in the study was taken from them according to the protocol approved by Universiti Sains Malaysia.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The research data supporting the findings of this study will be available upon request from corresponding author [N.Y].
FUNDING
This study was funded by Universiti Sains Malaysia, Research University Individual (RUI) Grant Scheme with Project No: 1001/PPSP/8012352, Project Code: UO1934 (Reference No: 2020/0202)
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank Universiti Sains Malaysia for the facilities and financial support.


