All published articles of this journal are available on ScienceDirect.
Mood-dependent Variations in Destination Memory: Evidence from an Experimental Study
Abstract
Introduction
While emotional influences on general memory are well-documented, the impact of mood on destination memory remains underexplored. This study investigates the effects of positive, negative, and neutral moods on destination memory performance.
Methods
Sixty participants from the Indian Institute of Technology Indore were randomly assigned to mood induction conditions and completed a destination memory task. The descriptive statistics were applied to check the central tendency of facts, faces, and videos, and the one-way ANOVA test was applied to test the hypothesis. We performed the Tukey HSD test to identify specific group differences. The valence of mood was an independent variable, and the scores of destination memory were a dependent variable.
Results
A one-way ANOVA revealed a significant effect of mood on performance [F (2, 57) = 5.25, p = .008, η2 = 0.13], with participants in a neutral mood performing significantly better than those in a positive mood. No significant difference was observed between negative and neutral conditions.
Discussion
The findings align with prior research suggesting that positive emotions broaden the attentional focus and promote relational processing, potentially leading to memory errors. In contrast, neutral moods provide an optimal cognitive state for accurate associative memory. These results contribute to the growing body of research on mood and memory, suggesting that positive mood impairs destination memory by increasing susceptibility to distraction and reducing cognitive control.
Conclusion
The study highlights the importance of considering emotional states in understanding social memory and suggests avenues for future research into the real-world implications of mood-related memory biases.
1. INTRODUCTION
Memory is one of the most fundamental aspects of human cognition, shaping how we navigate the world and interact with others. Among the many types of memory that contribute to our daily functioning, destination memory stands out as particularly important in the context of social interactions. Destination memory refers to the ability to remember to whom we have shared information [1]. For instance, recalling who you told about a recent event or whom you shared a piece of gossip with relies on this memory system. In daily life, destination memory is essential for effective communication and social functioning, allowing individuals to recall the recipients of their communications and maintain the coherence of their social exchanges. Despite its importance, the factors influencing destination memory have been relatively underexplored in comparison to other forms of memory, such as episodic or semantic memory. Destination memory plays a critical role in daily social interactions. While traditional memory research often focuses on memory for events or factual information, destination memory centers on the social aspect of memory. This process is essential in various social contexts, including interpersonal communication, group interactions, and professional settings. Errors in destination memory can lead to social awkwardness, misunderstandings, or the breakdown of communication.
Research shows that emotional stimuli are more likely to be remembered than neutral ones due to their ability to capture attention and enhance encoding, consolidation, and retrieval through mechanisms like amygdala activation [2]. However, when neutral information is learned in an affective state, it may be remembered less effectively because emotional processing biases attention toward emotionally salient details, impairing the encoding and integration of neutral information. This reflects the prioritization of emotionally relevant material over neutral content in memory systems [3]. Emotionally charged stimuli capture attention more readily during the perceptual process, which enhances the likelihood of encoding these stimuli into long-term memory (LTM). This selective attention is influenced by top-down control mechanisms in sensory pathways, which are regulated by the frontal and parietal cortices. These brain regions prioritize emotional information, guiding sensory processing and behavioral responses. As a result, emotionally relevant details are more likely to be stored in memory due to the enhanced attentional focus, demonstrating an indirect influence of emotions on perception and memory encoding [4, 5].
Mood is a pervasive and persistent feeling that can influence an individual's thoughts, behavior, and physiological responses. It is one such factor that affects cognitive abilities but has yet to be extensively examined in the context of destination memory. Unlike transient moods such as anger or joy, mood represents a more enduring emotional condition that typically lasts for an extended period, ranging from hours to days [6]. Previous research has classified mood into positive, negative, or neutral categories, with each influencing cognition in distinct ways [7, 8]. For instance, positive moods are often associated with greater cognitive flexibility and creativity [9, 10], while negative moods tend to narrow attention, fostering more focused and detailed processing [11]. Neutral moods, in contrast, are generally seen as baseline moods that do not induce significant emotional arousal or cognitive biases [12]. Several theoretical frameworks have been developed to explain how mood influences cognitive functions, particularly memory. One influential theory is the broaden-and-build theory of positive emotions, proposed by Fredrickson [13]. According to this theory, positive emotions broaden an individual’s cognitive and attentional scope, which enhances creativity, problem-solving, and the ability to integrate information. However, this broadening effect can also lead to a more general encoding of information, making it more difficult to recall specific details. On the other hand, negative moods have been associated with a narrowed focus. Negative emotions narrow attentional resources and focus cognitive processing on specific, goal-relevant information [11]. Negative emotions are thought to prioritize the processing of information that is immediately relevant to the individual’s goals, potentially enhancing memory for specific, social cues [14]. In a similar vein, Blaney (1986) found that positive mood facilitated the recall of more general, abstract memories, while negative moods improved recall of specific, detailed memories [15]. While positive and negative moods are often examined in isolation, it is also important to consider the role of neutral mood states, which serve as a baseline mood that does not induce significant cognitive biases [12]. Neutral mood states may therefore provide an optimal condition for encoding and recalling specific social information, as the individual’s attention is not diverted by emotional processing [16]. The influence of mood on memory is not limited to the initial encoding phase but also extends to the consolidation and retrieval stages [17, 18]. Research has shown that mood can affect how memories are consolidated, with positive moods potentially enhancing the consolidation of global, abstract aspects of memory and negative moods focusing on specific details [19]. A growing body of research has explored the relationship between mood and various types of memory. Much of the research on mood and memory has focused on broader memory systems, such as episodic memory and autobiographical memory. For example, Bower’s (1981) research demonstrated that mood-congruent memory retrieval is a common phenomenon, whereby individuals in positive moods tend to recall positive events and those in negative moods tend to recall negative events. This mood-congruence effect suggests that mood states can bias memory retrieval, making it more difficult to recall information that does not match the current mood. Neuroimaging studies indicate that the amygdala interacts with the hippocampus during emotionally arousing experiences, enhancing memory consolidation for emotionally charged events. This collaboration between the amygdala and hippocampus strengthens the encoding of emotional memories, making them more durable and accessible [20, 21].
Drawing on the Affect-as-Information theory, mood is posited to influence how information is processed: positive moods promote heuristic, less detail-oriented processing, whereas negative moods promote systematic, detail-focused processing. This suggests that mood may affect how well individuals encode or retrieve destination-related details. Furthermore, mood-congruent memory theory implies that the valence of information or context may interact with mood to shape memory outcomes. However, few studies have tested these theories specifically in the context of destination memory [22].
Research on destination memory and emotions has explored how emotions affect memory performance, particularly focusing on the mood of the recipient. Studies on patients with Korsakoff’s syndrome show a significant impairment in destination memory, especially for emotionally negative destinations. This suggests that individuals with Korsakoff’s syndrome have a diminished capacity to process negative emotional cues, which affects their ability to recall destination-related information [23]. In contrast, Alzheimer's Disease (AD) patients do not exhibit the same emotional effect on destination memory. Unlike healthy older adults, who tend to remember negative faces more accurately than positive or neutral ones, AD patients appear to show a generalized decline in emotional processing linked to destination memory [24]. For healthy older adults, emotional stimuli—particularly negative faces—are more likely to be remembered than positive or neutral stimuli, suggesting that emotional salience plays a crucial role in memory retention [1]. While providing valuable insights, these studies focus primarily on clinical and aging populations. This highlights a gap in the literature regarding how mood—particularly that of the sender—influences destination memory in younger, non-clinical populations. The effect of mood on the sender is yet to be systematically explored.
Emotional processing and memory are also deeply shaped by cultural norms, values, and identity, which influence both how emotions are experienced and how emotional events are remembered. Research across various cultural contexts has shown that emotional memory biases differ significantly: for example, while older adults in Western cultures often show a positivity bias in memory, East Asian older adults tend to recall both positive and negative information more equally, reflecting differing cultural attitudes toward aging and emotion [25]. Similarly, cultures that emphasize emotional regulation, such as many East Asian and Middle Eastern societies, may exhibit reduced emotional vividness in memory compared to Western cultures that promote emotional expression [26]. These differences extend to autobiographical memory and social memory processes, which are constructed in ways that align with culturally valued goals and social relationships. The value-congruence model further explains how emotional events that align with cultural values are remembered more vividly [27]. Moreover, mood induction paradigms—such as those using emotionally evocative video clips—have been successfully validated across diverse countries, supporting the global applicability of such methods while also acknowledging that cultural background may shape the strength and nature of induced mood states [28]. Taken together, these international findings highlight the culturally embedded nature of mood and memory processes, and underscore the need to study destination memory within a cultural context—given that both memory and emotion are shaped by cultural norms, and destination memory itself is a fundamentally social form of memory.
Given the limited research on mood and destination memory, the present study seeks to examine how positive, negative, and neutral moods influence performance on a destination memory task. The findings of this study could have important implications for understanding how mood influences social cognition and memory, providing new insights into how emotions affect the accuracy of social interactions and communication. By investigating the relationship between mood and destination memory, this research aims to contribute to the broader field of memory research and offer practical insights into the role of emotions in everyday social functioning.
2. MATERIALS AND METHODS
2.1. Study Design
This study aimed to examine how different mood states—positive, negative, and neutral—affect destination memory performance. The research question guiding this investigation was: “Does the mood of the information sender influence their ability to recall to whom information was shared (destination memory)?” To address this, a quantitative, analytical study design was employed.
2.2. Search Strategy
To inform the study’s rationale and design, a narrative review of the literature was conducted using databases such as Google Scholar, PubMed, PsycINFO, and Scopus. Search terms included combinations of “destination memory,” “mood and memory,” “emotional interference,” and “associative memory.” Peer-reviewed articles published in English and focusing on the cognitive effects of mood or emotion on memory were included. Clinical or animal studies and non-peer-reviewed sources were excluded. Studies were selected based on relevance to the topic and contribution to theoretical and empirical understanding. Key findings were synthesized to guide hypothesis formulation and experimental design. The results of this literature search and the accompanying narrative synthesis are presented in the Introduction section to provide the context and rationale for the study design and hypotheses.
2.3. Participants
The study included 60 (male: 45; female: 15) participants recruited from the Indian Institute of Technology Indore’s student population. Participants ranged in age from 18 to 30 (Mean age: 20.9 ± 2.4) years (Table 1). Sample size was determined via a priori power analysis (G*Power 3.1) for a one-way ANOVA, indicating 60 participants total (20 per group). They were randomly assigned to one of three mood induction groups: positive, negative, or neutral. All participants had normal or corrected-to-normal vision and were screened for any history of neurological or psychiatric disorders. They volunteered for the study by filling out a circulated Google form. The participants received a monetary reward as per the IHEC (Institute Human Ethics Committee) norms. The IHEC of IIT Indore approved this study.
| Variable | Value |
|---|---|
| Total Participants | 60 |
| Gender | Male: 45 (75%) Female: 15 (25%) |
| Age (years) | M = 20.9, SD = 2.4, Range: 18–30 |
2.4. Materials Required
We selected universal facts and faces from a database and videos from YouTube as stimuli for the experiment, which were then validated through participant ratings.
2.5. Facts Used
The stimuli included facts that were validated to be familiar to most raters. The first 60 universal or most likely known facts by the Indian population were chosen. Then these facts were rated based on their familiarity by participants through a Google form. For the rating of the facts as either familiar or unfamiliar, the participants had to rate the facts as “0” (unfamiliar) or “1” (familiar). Out of the total facts rated those with the highest familiarity scores were used. We used a total of 40 most familiar facts out of the 60 facts.
2.6. Faces Used
We used 40 neutral images of people from the KDEF (Karolinska Directed Emotional Faces) database [29], which consists of standardized emotional expressions from individuals who provided informed consent for non-commercial research use. The images are high-resolution (1024×768 pixels) and are formatted in JPEG or BMP. The images are presented in full color. The faces were validated using a 3-point rating scale, where 1 represented negative, 2 represented neutral, and 3 represented positive.
2.7. Videos Used
A total of twelve videos were sourced from YouTube for mood induction, out of which three videos were selected for the final experiment and rated by participants on the SAM (Self-Assessment Manikin) rating scale [30]. The videos were rated for valence (negative-positive), arousal (excited-calm), and dominance (dependent-independent) dimensions. We used a total of three videos for the experiment, one from each valence; each had an average playback time of 2:13 minutes.
Before the experiment, we conducted the Mini-Mental State Examination, a general questionnaire, a mood questionnaire, and a memory and attention test to meet the inclusion criteria.
2.8. Procedure
Prior to participation, all individuals were provided with a written informed consent form detailing the nature and purpose of the study, including the experimental procedures, their right to withdraw at any point without penalty, and the assurance of confidentiality and anonymity of their data. Participants were instructed to read the form carefully and were given the opportunity to ask questions. Only those who voluntarily agreed and signed the consent form were included in the study. Along with that they filled the other questionnaires mentioned above. The whole experiment was divided into three phases, viz., mood induction, learning (of facts and faces), and test phases. We presented all the stimuli using the stimulus presentation software E-Prime version 3.0 [31].
2.9. Mood Induction Phase
In the first phase, participants sat in the experiment room and watched a video. After watching the video, they had to fill out the SAM rating scale.
2.10. Learning Phase
In this phase, the participants were shown a fact (Fig. 1); after reading the fact, they were instructed to move to the next slide by pressing the spacebar, after which a photo of a person was shown. They had to say the fact aloud to the face. Once they had said the phrases to the faces, they would press the spacebar to move to the next slide. We instructed them to remember which face they had spoken the phrase to.
2.11. Test Phase
During the test phase, the participants had to perform a recognition task. They would first see the fact and were asked if they remembered seeing the fact before. In the next slide, a photo of a person was shown, and were asked whether they remembered saying the fact to that person. They would then have to click on the ‘yes’ or ‘no’ options depending on whether they remembered telling the fact to the photo of the person (Fig. 2). We introduced a distractor task between the learning and test phases.
2.12. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 27 (Statistical Package for Social Sciences). Descriptive statistics were applied to check the central tendency of facts, faces, and videos, and the one-way ANOVA test was applied to test the hypothesis. We performed the Tukey HSD test to identify specific group differences. The valence of mood was an independent variable, and the scores of destination memory were a dependent variable.
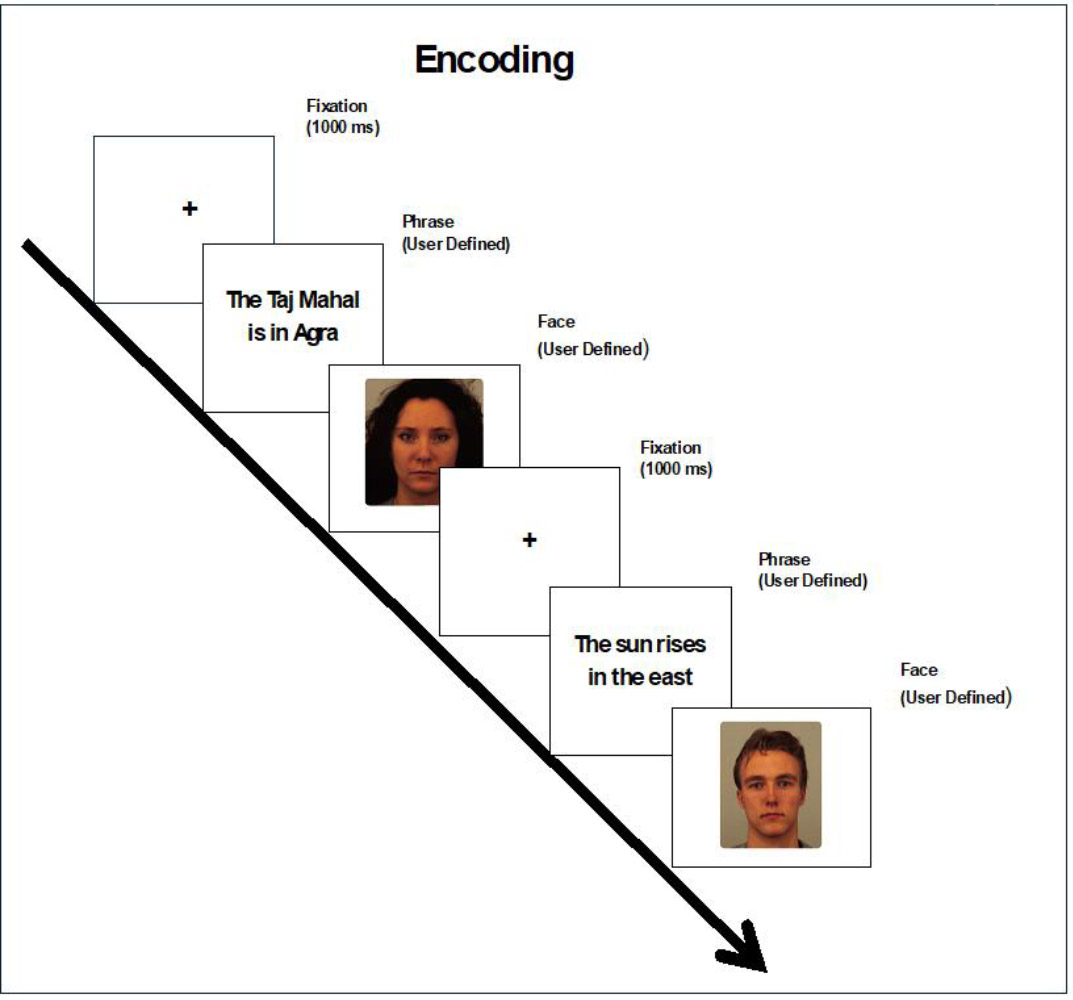
Encoding paradigm with sample KDEF stimuli (AF02NES). Images used with participant consent [29].
3. RESULTS
3.1. Descriptive Statistics
The mean and standard deviation values were calculated for facts, faces, and video clips. We selected a total of 40 facts, rated on a familiarity scale from 0 (unfamiliar) to 1 (familiar), yielding high familiarity overall (M = 0.96, SD = 0.04). Forty faces were rated on a 3-point scale, where 1 indicated negative, 2 neutral, and 3 positive valences. The mean rating across face categories was M = 2.00 (SD = 0.05), suggesting a balanced distribution across emotional expressions.
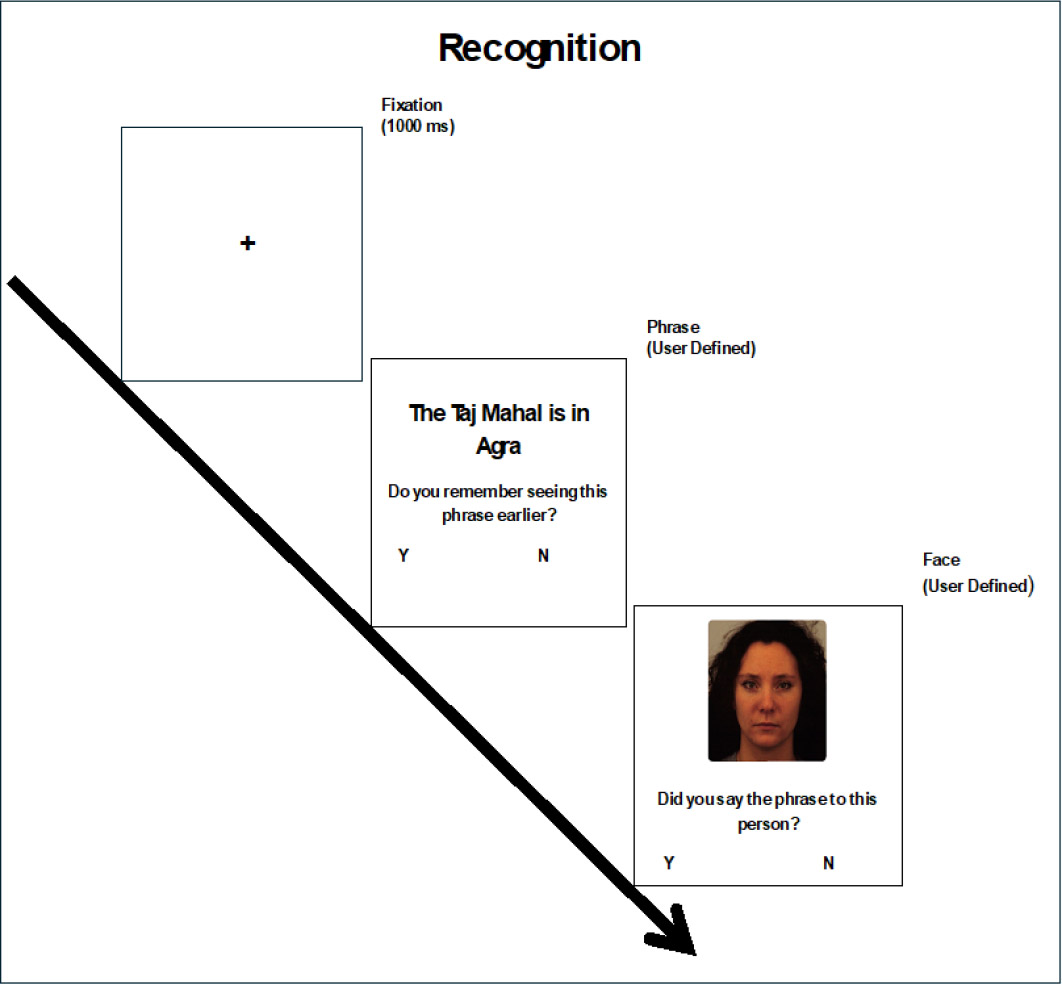
Recognition paradigm with sample KDEF stimuli (AF02NES). Images used with participant consent [29].
Induced mood ratings were obtained using the Self-Assessment Manikin (SAM) scale across three emotional dimensions: valence, arousal, and dominance. As shown in Table 2, positive and negative videos were rated similarly high in arousal (M = 4.35, SD = 0.74 and M = 4.30, SD = 0.97, respectively), while neutral videos had substantially lower arousal (M = 1.25, SD = 0.55). A similar pattern was observed for dominance: both emotional video types scored high (positive: M = 4.25, SD = 0.85; negative: M = 4.10, SD = 1.02), while neutral videos were rated low (M = 1.25, SD = 0.44). In terms of valence, the ratings followed the expected emotional trajectory—positive videos received the highest valence scores (M = 4.55, SD = 0.75), negative videos the lowest (M = 1.55, SD = 1.05), and neutral videos fell in between (M = 3.10, SD = 0.30) (Table 2).
3.2. Inferential Statistics
Descriptive statistics for destination memory (DM) performance across mood conditions are presented in Table 3. Participants in the neutral mood condition demonstrated the highest mean DM score (M = 13.70, SD = 2.84), followed by those in the negative mood condition (M = 11.85, SD = 3.01), and the lowest performance was observed in the positive mood condition (M = 10.90, SD = 2.44). This pattern suggests that a neutral emotional state may facilitate better memory for social information (i.e., who said what), compared to both positive and negative moods.
| - | Negative | Positive | Neutral |
|---|---|---|---|
| Arousal | 4.30 ± 0.97 | 4.35 ± 0.74 | 1.25 ± 0.55 |
| Dominance | 4.10 ± 1.02 | 4.25 ± 0.85 | 1.25 ± 0.44 |
| Valence | 1.55 ± 1.05 | 4.55 ± 0.75 | 3.10 ± 0.30 |
| Mood Conditions | Mean Score of DM | Std. Deviation |
|---|---|---|
| Negative | 11.85 | 3.01 |
| Positive | 10.90 | 2.44 |
| Neutral | 13.70 | 2.84 |
To statistically examine these differences, a one-way ANOVA was conducted with mood (positive, neutral, negative) as the between-subjects factor. The analysis revealed a significant main effect of mood on destination memory performance, F (2, 57) = 5.25, p = .008, η2 = 0.13, indicating a moderate effect size. Post hoc comparisons using the Tukey HSD test showed that neutral mood participants significantly outperformed those in the positive mood condition (p = .007). However, no significant differences were observed between the neutral and negative mood groups (p = .098) or between positive and negative mood groups (p = .530).
4. DISCUSSION
This study assessed the effect of the participants’ mood (neutral, positive, and negative) on the destination memory performance. Our results revealed that destination memory performance was better in a neutral mood than in a positive mood. Similarly, individuals in a negative mood performed better than those in a positive mood; however, performance in the negative mood was less than in the neutral mood, though these differences were not statistically significant. Overall, the findings indicate that destination memory performance is impaired in a positive mood compared to a neutral mood.
Some previous research has widely supported the idea that emotions enhance memory. This phenomenon is called the Emotion-enhanced memory (EEM). EEM refers to the robust phenomenon where emotionally charged stimuli are more likely to be remembered than neutral ones [32, 33]. However, this effect has been seen with emotional stimuli that are presented to the participant, rather than the affective state of the individual. Our results align with research showing that emotions enhance memory in general but do not improve, and may even impair, associative memory [34, 35]. Research consistently shows that emotional items are associated with poorer detailed memory for accompanying scenes and reduced recognition accuracy for object-scene pairings or item pairs. This is also called ‘emotional interference’ [36-39]. Additionally, in a study studying the effect of arousal on temporal binding, the level of binding was found to be generally weaker for high-arousal images compared to low-arousal ones [40]. This suggests that the intensity of emotional stimulation might interfere with the cognitive processes involved in binding, potentially due to the heightened distraction or attentional demands associated with high-arousal stimuli. This could explain why participants in our study in the neutral mood group performed better as compared to the positive and negative affect groups. In contrast, low-arousal images may allow for more focused cognitive engagement, facilitating stronger associations or connections. Similarly, research investigating the influence of emotional content on item and associative memory revealed that while emotion enhances item memory, it has a detrimental effect on associative memory. This suggests that emotional arousal may differentially affect cognitive processes, bolstering memory for individual items while impairing the ability to form and retrieve associations between them [41]. While these prior studies focused on emotional stimuli that directly engage attention and cognitive resources, the present study demonstrates that induced moods can similarly disrupt associative memory, albeit through different mechanisms. Positive mood, associated with relational and heuristic processing, may have led participants to over-rely on schemas and failure to encode specific source-destination pairings. Conversely, negative mood, while fostering item-specific processing, may have shifted attention to individual details at the expense of forming robust associations. Neutral mood, free from emotional or cognitive interference, appears to provide an optimal baseline for destination memory, facilitating the balance of encoding and retrieval processes necessary for accurate performance [12]. This parallels the role of neutral stimuli in prior research, further suggesting that emotional states—whether evoked by stimuli or induced as moods—introduce biases that impair associative memory.
In our study, positive mood was found to impair destination memory performance the most. This can be attributed to several factors. The first explanation is the broadening effect of positive emotions on our cognition. It claims that positive emotions expand the thoughts, impulses to act, and perceptions that naturally arise in comparison to negative emotions and neutral states. It is called the broaden-build theory [42]. In one of the first experiments of its kind, their team compared the performance of participants on a global-local visual processing task of participants in positive, negative and neutral moods. Previous research found that negative traits like anxiety are linked to local focus, while positive traits like optimism favor broader perspectives [43]. The results reiterated these findings and revealed that participants in positive moods (amusement and contentment) selected the global option more than the neutral group [44]. Another study also found that people in a positive mood had more attentional flexibility than those in a negative mood [45]. Individuals in a positive mood have top-down processing, whereas negative moods induce bottom-up processing. Top-down processing promotes creativity and relational thinking, which can result in a broader memory but also more errors of memory, whereas bottom-up processing relies on directed and detailed thinking, which results in more accurate memory.
Another explanation for the weaker performance in a positive mood is that positive emotions are less susceptible to inhibitions when compared to negative emotions, especially under restricted attentional resources [46-49]. Similarly, in high vs low load conditions, it was found that higher load conditions of a primary letter search task reduced interference from negative distractors but did not affect interference from positive distractors [46]. Given that the destination memory task demands full attention to recall associations between information and the face it was shared with, positive moods may cause greater interference compared to negative moods. Given that the destination memory task demands full attention to recall associations between information and the face it was shared with, positive moods may cause greater interference compared to negative moods. This highlights the critical role of attentional control in associative memory tasks like destination memory and underscores how mood-induced differences in susceptibility to distraction can directly impact performance.
Along with that, positive stimuli also have a unique capacity to engage sustained neural activity across both visual and emotional processing areas, such as the ventral visual cortex, fusiform gyri, ventral striatum, and amygdala [50, 51]. This prolonged activation ensures that positive stimuli are processed with heightened attention and detail, leading to the formation of strong and vivid mental representations. Such sustained engagement is thought to amplify the salience of positive experiences, making them more memorable and emotionally impactful. However, this heightened focus on positive stimuli can also create an attentional bias, where cognitive resources are disproportionately allocated to processing these rewarding experiences at the expense of other contextual or peripheral details. This bias reflects a prioritization mechanism in the brain, where emotionally uplifting or rewarding information captures attention and is encoded more robustly than neutral or negative stimuli. While this may enhance memory for the positive stimulus itself, it can inadvertently lead to reduced attention or memory of information being encoded after the positive affective state is induced using the stimuli. This, in our case, explains the weak destination memory performance.
An additional perspective for the positive affect group’s weaker performance could be the distracting characteristic of positive emotions [52]. Dopamine, a neurotransmitter crucial for reward processing, plays a significant role in redirecting attentional resources. Positive emotions are found to be more distracting than negative emotions because they inherently engage reward-related dopaminergic pathways, which prioritize rewarding stimuli over task-relevant information [53]. Positive emotions, being naturally rewarding, activate these pathways more strongly than negative emotions, leading to an involuntary capture of attention by positive distractors [54]. Unlike negative emotions, which often trigger heightened vigilance or a need for caution (potentially aiding task focus), positive emotions can reduce motivation or ability to control distraction. Participants are less likely to exert cognitive control to resist the allure of positive stimuli because of the inherent reward they provide. This effect is amplified by dopamine's role in shifting attention from goal-directed tasks toward rewarding or pleasurable stimuli. This could explain the positive affect group’s poorer performance in destination memory, as the task requires sustained attention to form and retrieve associations between people and information, which may be disrupted by the attentional shifts caused by a positive mood.
These findings have important implications for how mood states impact social communication and memory in daily life. Destination memory, by nature, is a socially embedded form of memory that helps individuals track what information they have shared with whom. Impairments in destination memory during positive mood states, as revealed by our study, may have real-world consequences—for example, forgetting whether one has already shared certain information in conversations, academic discussions, or professional contexts. This suggests that while positive moods are often associated with cognitive benefits such as creativity and flexibility, they may also lead to reduced attention to detail and weaker encoding of associative links critical for social memory. The results also offer a nuanced perspective on the emotion-enhanced memory literature, showing that mood states, unlike emotional stimuli, do not universally enhance memory and may have domain-specific effects. Our findings highlight the need to examine how both transient and prolonged mood states influence communication-based memory processes. Future research could build on this by exploring destination memory in naturalistic and culturally diverse settings, or in populations experiencing persistent mood changes, such as those with mood disorders. This would further clarify the role of emotion in everyday memory functioning and social cognition.
5. LIMITATIONS
While this study provides valuable insights into the relationship between mood and destination memory, it is not without limitations. First, the sample consisted predominantly of male and female university students from a single institution (IIT Indore), which may limit the generalizability of the findings across gender, age, and cultural contexts. Given that emotional processing and memory can differ across demographic groups, future research should recruit more diverse and gender-balanced samples. Second, although mood was successfully induced through validated video stimuli in a controlled lab setting, such methods may not fully replicate the complexity of real-world emotional experiences. Naturally occurring moods—such as stress at the workplace or joy from social media interactions—may influence destination memory differently than experimentally induced affect. Future studies employing ecologically valid designs (e.g., daily diary methods or ambulatory mood tracking) are needed to explore destination memory in everyday life. Moreover, while this study focused on transient, situational mood effects, future research could investigate how prolonged or clinical mood states (e.g., depression, anxiety, PTSD) modulate destination memory over time. This would be particularly valuable given the importance of memory disturbances in many affective disorders. Moreover, the destination memory task employed in this study involved static associations between faces and facts, which may not reflect the interactive nature of real-life conversations. Destination memory in natural settings often includes dynamic social cues such as speech, gestures, and feedback from others. Future research could explore more ecologically valid paradigms, such as live or virtual social interactions, to enhance generalizability. Additionally, while mood induction was verified initially via self-report, we did not assess whether the induced mood persisted throughout the memory task. Future studies should include repeated mood assessments or physiological indicators to better track mood stability and its effects on encoding and retrieval phases separately.
CONCLUSION
In conclusion, the findings of this study shed light on the nuanced relationship between moods and destination memory performance. These findings suggest that a positive mood is more likely to impair destination memory performance than a neutral mood. This underscores the complexity of emotional influences on cognitive processes and highlights the need for further research to explore how different moods modulate various aspects of memory.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: T.R., K.V.: Study conception and design; T.R., G.J.: Data collection; T.R., K.V.: Analysis and interpretation of results; T.R.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ANOVA | = Analysis of Variance |
| LTM | = Long Term Memory |
| AD | = Alzheimer’s Disease |
| IHEC | = Institute Human Ethics Committee |
| KDEF | = Karolinska Directed Emotional Faces |
| JPEG | = Joint Photographic Experts Group |
| BMP | = Bitmap |
| SAM | = Self-Assessment Mankin |
| EEM | = Emotion-Enhanced Memory |
| SPSS | = Statistical Package for Social Sciences |
| M | = Mean |
| SD | = Standard Deviation |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval was obtained from IHEC (Institute Human Ethic Committee) at Indian Institute of Technology Indore, India (Approval Date: July 19, 2023, Reference No.: BSBE/IITI/IHEC-09/2023).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All participants provided informed consent before participating in the study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [K.V] upon reasonable request.
ACKNOWLEDGEMENTS
The authors wish to thank all participants for their valuable contributions to this study.


