RESEARCH ARTICLE
Post-extinction Delay Necessary to Induce Retrograde Amnesia for a Moderate Extinction Training Memory
Ashlyn J. Zikmund, James F. Briggs*
Article Information
Identifiers and Pagination:
Year: 2016Volume: 9
First Page: 66
Last Page: 74
Publisher ID: TOPSYJ-9-66
DOI: 10.2174/1874350101609011866
Article History:
Received Date: 9/6/2016Revision Received Date: 4/7/2016
Acceptance Date: 12/7/2016
Electronic publication date: 29/07/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Two experiments using rats were conducted to evaluate the post-extinction delay necessary to obtain retrograde amnesia for a moderate extinction training memory. In Experiment 1, six minutes of extinction (i.e., cue-exposure) was sufficient to reduce fear of the black compartment of a white-black shuttle box, however the amnestic treatment cycloheximide (CHX) failed to produce retrograde amnesia for extinction (i.e., show fear). In Experiment 2, CHX was administered at various post-extinction delays (0-min, 60-min, 75-min, 120-min) to assess whether the active extinction memory could be susceptible to amnesia if the original fear memory had time to reconsolidate. The results indicated that administrating CHX 75 minutes after extinction produced retrograde amnesia for extinction, but not for shorter post-extinction delays, thus demonstrating a temporal gradient. These findings suggest that the extinction memory was active and susceptible to disruption 75 minutes after the extinction session, but the original fear memory may have been protected from the amnestic effects with sufficient time to reconsolidate.
INTRODUCTION
The time-dependent nature of retrograde amnesia in conjunction with the consolidation theory has been studied extensively over the years [1-3]. Research supporting the consolidation theory suggests that newly formed memories are susceptible to disruption until they are consolidated, whereas older, more mature memories are protected and remain intact [4-6]. Although the consolidation view proposes older memories resist disruption, a growing body of research suggests the level of activation is of greater importance than the age of the memory in terms of the susceptibility to amnesic agents. While early investigations supported the consolidation theory, there is evidence demonstrating that previously stored memories can become labile and susceptible to disruption after being reactivated [7-9]. This retrieval failure view of retrograde amnesia hypothesizes that when a memory is reactivated it is once again in an active and labile state vulnerable to disruption, thus the memory must reconsolidate before it is no longer susceptible to disruption [10-12].
The phenomenon referred to as reconsolidation of a reactivated memory has been a topic of increasing interest. For example, studies by Nader, Schafe, and LeDoux [9] as well as Land, Bunsey, and Riccio [13] have established that reactivated memories are susceptible to disruption by protein synthesis inhibitors (PSI) as well as lesions to the hippocampal area, respectively. In addition, it has been demonstrated that inhibiting protein synthesis impairs the reconsolidation of memories for conditioned taste aversion [14], appetitive learning tasks [15], as well as spatial memory tasks [16].
Memories for extinction (i.e., cue exposure) have also been shown to be susceptible to retrograde amnesia using a number of amnestic agents, including PSIs [17-20]. Extinction involves exposure to cues (conditioned stimuli) that have been previously paired with a biologically relevant reinforcer (unconditioned stimulus) without the outcome, which leads to a reduction in responding. It is well established that extinction involves new learning, rather than a breakdown of the original association [21, 22] and it has been shown that retrograde amnesia for extinction shares similar characteristics to amnesia for original acquisition memories [23].
Moreover, similar to the importance of the activity level with regard to susceptibility of a newly formed memory, the duration of extinction training (i.e., cue exposure) is a factor in obtaining amnesia for extinction using PSI’s while preserving the original memory. For example, using a contextual fear paradigm Suzuki, Josselyn, Frankland, Masushige, Silva, and Kida [24] showed that short amounts of contextual exposure (0, 1, or 3 minutes) did not produce extinction but served as reminders, leaving the reactivated memory vulnerable to the PSI anisomycin. On the contrary, a longer 30-minute exposure did produce extinction, rendering extinction susceptible to amnesia, leaving the original fear memory intact. That is, with short exposure durations amnesia for a reactivated memory was obtained, however with longer exposure amnesia for extinction was observed [see also 18, 19, 25, 26]. Thus, it appears that the dominant trace active during the time of amnestic treatment is the important factor in determining amnesia.
Evidence for the extinction of fear as new learning supports that these two processes are separate events and can be thought of as forming distinct “competing” memories while they are both actively encoded [see 25]. Currently, there is little known regarding the interaction of these competing memories, particularly after post-extinction delays. It is hypothesized that during the extinction session the original acquisition memory will be reactivated and reconsolidated while extinction learning is simultaneously being encoded and stored. Over the time, the newly acquired extinction memory will continue to consolidate after the original has fully reconsolidated following reactivation, leaving only the extinction trace vulnerable to disruption [see 1]; however, the temporal gradient of the competing memories vulnerability remains unclear. Thus, the aim of the present studies is to investigate when an extinction memory becomes the dominant trace, leaving it susceptible to retrograde amnesia using the protein synthesis inhibitor cycloheximide (CHX). In order to obtain the amnestic effects of the competing memory, the extinction memory must be the active dominant trace at the time the amnesic treatment is administered; therefore, the temporal gradient of competing memories will be investigated by administering CHX at various post-extinction delays, rather than extending the extinction sessions as was the focus of previous research.
Experiment 1
This experiment served to determine a moderate amount of extinction training that would reduce fear, while also be short enough in duration that it would allow the original acquisition memory to be actively reconsolidating and vulnerable to disruption. Previous research from our lab has shown that 6 minutes of extinction following punishment training reduced fear, although failed to obtain amnesia for extinction (i.e., a return of the fear memory) in a passive avoidance paradigm [18]. Although the extinction duration gained behavioral control, the original fear memory appeared to be in an active state and remained vulnerable to CHX. Thus, a 6-minute exposure session was used to accomplish retrograde amnesia for both memories when the amnestic agent was administered immediately following extinction, replicating our previous results. It was hypothesized that the 6 minutes of extinction would not be a sufficient amount of time to allow the original fear memory to be reconsolidated and protected from disruption, leaving both memories in an active and vulnerable state. Thus, we expected to observe little fear in the amnestic CHX group because of induced retrograde amnesia of the original fear memory.
METHODS
Subjects
Twenty-four experimentally naïve, female Long-Evans hooded rats, obtained from Susquehanna University’s animal facility breeding colony, served as subjects. The subjects were approximately 95 days old at the start of the experiment and had an average weight of 280 grams. The rats were individually housed and had free access to food and water, and were maintained on a 12-hour light-dark cycle. All experimental sessions occurred during the light cycle, and were done at the same time each day. Approval of the experimental protocol was obtained by the Susquehanna University Institutional Animal Care and Use Committee prior to data collection.
Apparatus
Training, extinction, and testing were conducted in a 48 x 21 x 21 cm white-black passive avoidance chamber (Ugo Basile Model 7550) with a grid floor. The chamber was divided into two equal compartments, a white compartment and a black compartment. An automatic sliding door that allows the rat access to pass from one compartment to the other separated the two compartments. The white compartment had opaque white surrounding walls with a transparent lid, whereas the black compartment had both opaque black walls and lid. A shock was delivered automatically through the grid floor of the black compartment via a controller panel.
Procedure
Prior to the beginning of the experiment, all rats were handled for 5 minutes on two consecutive days before being weighed and randomly assigned to one of the four groups. Twenty-four hours after handling, each rat received a single fear conditioning trial. During fear conditioning, each rat was placed in the white compartment of the white-black shuttle box facing away from the closed door. After 15 seconds, the door opened and the latency to cross into the black compartment (tilted floor) was automatically recorded. Upon entering the black compartment the door automatically shut and, after a 2 second delay, the rat received one inescapable foot shock (1 second, .8 mA). Fifteen seconds after the door was shut, the rat was removed from the apparatus and returned to its home cage. A fear only group (Fear) received only fear conditioning and no extinction to serve as a control showing fear of the black compartment.
Twenty-four hours after conditioning, three groups received a single cue-exposure (i.e., extinction) session. During this trial, rats were first placed in the white side of the black-white chamber for 15 seconds and then in the black compartment for 6 minutes. There were no shocks delivered during extinction and the sliding door remained closed, preventing the rats from crossing between compartments. One extinction group (Extinction) served as an extinction control group in order to show that 6 minutes was sufficient to extinguish the fear behavioral response. The parameters chosen for extinction were similar to a previous study done in our laboratory [18], which found that 6 minutes of extinction reduced fear of the black compartment. Immediately following the extinction session, a second extinction group (CHX) received an injection of CHX (1 mg/kg, i.p.). This group served as an amnesia control group to show retrograde amnesia for the reactivated fear memory as well as the extinction memory. To assess the contribution of the stressful injection, a third extinction vehicle-injection control group (Saline) received an equivalent volume (1 mg/kg, i.p.) injection of physiological saline (0.9%) immediately after extinction. This Saline control group also controlled for the possibility that the injection alone acted as a disinhibitor to produce recovery from extinction. The experimental design is summarized in Table 1.
Experimental design for Experiment 1.
| Group | Training | -24 hr- | Extinction |
Amnesia Treatment |
-24 hr- | Test |
|---|---|---|---|---|---|---|
| Fear | Yes | No | No | Yes | ||
| Extinction | Yes | Yes | No | Yes | ||
| CHX | Yes | Yes | Yes | Yes | ||
| Saline | Yes | Yes | Saline | Yes |
Twenty-four hours after extinction (48-hours after initial fear conditioning for the Fear group), all animals underwent passive avoidance testing. Test trials were conducted identically to training trials, except no shocks were delivered upon the rat’s entrance to the black compartment. After crossing to the black compartment, the door automatically closed and the rat was removed from the shuttle box and placed in its home cage. Rats that did not cross to the black compartment and thus remained in the white compartment were removed from the white side after 540 seconds. The latency to cross to the black side of the shuttle box was recorded in seconds as the dependent measure.
RESULTS
Training
All four groups had short cross latencies at training with group means ranging from 19.9 to 28.2 seconds. An ANOVA revealed that there were no significant differences among the groups, F (3, 20) = .166, p = .92.
Testing
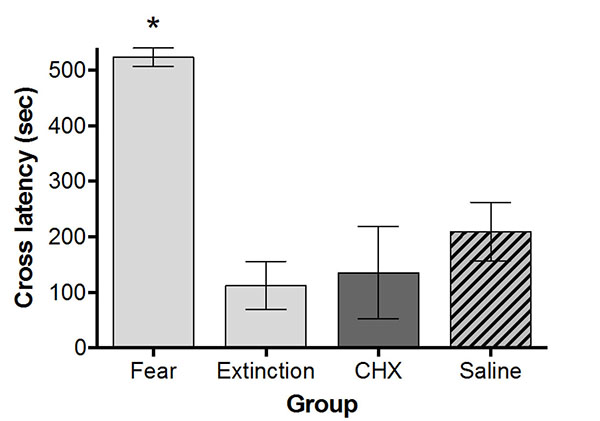
Fig. (1) shows the mean cross latencies for all four groups in Experiment 1. An ANOVA revealed that the groups differed significantly, F (3, 20) = 12.29, p < .001. Dunnett’s multiple comparison tests were used to compare each group to the Extinction control. As can be seen, the Fear group showed long avoidance scores (more fear) and all groups that received extinction exhibited less fear than the Fear group, and the Extinction and Fear groups differed significantly (p < .001), which demonstrates the moderate 6 minutes of extinction was sufficient in reducing fear. In addition, the Extinction group had similar short cross latencies to the CHX group (p = .94) and the Saline group (p = .46). Thus, the CHX did not produce retrograde amnesia for extinction. These results are somewhat difficult to interpret since it is not clear whether the PSI had no effect on extinction or whether the CHX induced retrograde amnesia for the reconsolidation of the original fear memory. We speculate the latter, that the amnestic agent produced retrograde amnesia for both the reconsolidated original memory and the extinction memory since both were active at the time of amnestic treatment. Experiment 2 was designed to test whether we were correct in our assumption.
Experiment 2
The results of Experiment 1 demonstrate that although 6 minutes of extinction was sufficient to reduce fear, retrograde amnesia appeared to be obtained for the reactivated fear memory and not the extinction memory alone. Although problematic, these results are consistent with what others have found [18, 19, 24-26]. The aim of Experiment 2 was to then investigate whether a post-extinction delay would allow sufficient time for the reactivated fear memory to reconsolidate, become a separate compartmentalized trace, and not be susceptible to amnesia, while leaving the extinction memory active and vulnerable to disruption. We hypothesized that CHX injections following post-extinction delays would allow time for the fear memory to reconsolidate, while leaving the recently formed extinction memory vulnerable to disruption. Therefore, we expected to obtain amnesia for extinction (i.e., more fear) following longer post-extinction CHX injection delays.
METHODS
Subjects and Apparatus
Fifty-six experimentally naïve (28 male and 28 female) Long-Evans hooded rats were used as subjects. Females weighed approximately 265 grams, males weighed approximately 448 grams, and all rats were approximately 100 days old at training day. An equal number of male and female rats were randomly assigned to one of seven groups. Animals were obtained and housed in the same manner as in Experiment 1, and the same apparatus was used.
Procedure
All handing, training, extinction, CHX injections, and testing methods were identical to Experiment 1. Differences in experimental manipulation are noted.
Before training, the rats were randomly assigned to one of the seven groups. As in Experiment 1, all rats received fear conditioning 24 hours after handling. One fear control group (Fear) received only fear conditioning. Twenty-four hours after fear conditioning, six groups received a single extinction session.
One extinction control group (Extinction) received only a single extinction session 24 hours after fear conditioning. Following the extinction sessions, four experimental CHX groups (0-min, 60-min, 75-min, and 120-min) received the amnesic treatment either immediately, 60 minutes, 75 minutes, or 120 minutes after extinction, respectively, to assess when the extinction memory gained behavioral control. These delays were chosen from past research demonstrating a temporal gradient for amnesia for extinction [23]. A vehicle-injection control group (0-min) received an injection of equivalent saline immediately following extinction. Twenty-four hours after the extinction session (48 hours after training), all seven groups underwent passive avoidance testing. The design of Experiment 2 is summarized in Table 2.
Experimental design for Experiment 2.
| Group | Training | -24 hr- | Extinction | Delay |
Amnesia Treatment |
-24 hr- | Test |
|---|---|---|---|---|---|---|---|
| Fear | Yes | No | ----- | No | Yes | ||
| Extinction | Yes | Yes | ----- | No | Yes | ||
| 0-min | Yes | Yes | 0 min | Yes | Yes | ||
| 60-min | Yes | Yes | 60 min | Yes | Yes | ||
| 75-min | Yes | Yes | 75 min | Yes | Yes | ||
| 120-min | Yes | Yes | 120 min | Yes | Yes | ||
| 0-min | Yes | Yes | 0 min | Saline | Yes |
RESULTS
Training
All seven groups exhibited short cross latencies at training ranging from 20.5 to 38.9 seconds. There were no sex differences in training cross latencies, t (54) = .01, p > .99, thus the sexes were combined for analysis. An ANOVA revealed there were no significant differences among the cross latencies of the seven groups during training, F (6, 49) = .48, p = .82.
Testing
Fig. 2 shows the mean cross latencies for all seven groups in Experiment 2. There were no sex differences in testing cross latencies, t (54) = .06, p = .96, thus the sexes were combined for all analyses. An ANOVA revealed that there was an overall difference among the seven groups, F (6, 49) = 5.84, p < .001. Dunnett’s multiple comparison tests were used to compare each group to the Extinction control. As can be seen, Dunnett’s multiple comparison tests confirmed that the Fear group differed significantly from the Extinction group (p = .001). The Dunnett’s tests also revealed that there were no significant differences between the Extinction group and the 0-min (p = .42) and 60-min groups (p = .12), demonstrating the CHX may have had an effect on both the reconsolidation of the reactivated memory and the extinction memory. Importantly, the post-hoc tests revealed that the 75-min group differed significantly from the Extinction group (p = .03). Thus, with a longer post-extinction delay retrograde amnesia for the extinction memory was observed while the original memory remained stable and not affected. Moreover, the 120-minute group did not differ from the Extinction group (p = .99). Thus, it appears that with the longer 120-minute post-extinction injection delay both the original and the extinction memories were reconsolidated and consolidated, respectively, thus inactive and not vulnerable to disruption. Although this long post-extinction delay group received the same treatment as the other CHX groups less fear was observed, which resulted in a fear level similar to the extinction only group. In addition, the 0-min saline vehicle control group was not significantly different from the Extinction group (p = .99). This group also demonstrated that the injection itself did not result in a change in performance.
DISCUSSION
The results of Experiment 1 show that although a moderate amount of extinction (6 minutes) is sufficient to reduce fear, retrograde amnesia for extinction was not obtained when CHX was administered immediately after the extinction session. We speculate that the failure to obtain amnesia for extinction in Experiment 1 was in part due to insufficient time to allow the reactivated fear memory to fully reconsolidate. This is shown by the amnesic effects of an immediate injection of CHX appearing to disrupt both the reactivated original memory, as well as the consolidation of the new extinction memory.
These results are difficult to interpret, as it is unclear whether the amnesic agent had no effect on either memory trace, had no effect on the extinction memory, or had an effect on both memories. In all three scenarios, the results would be similar, i.e., little fear following the injection after extinction training. However, previous findings have shown that CHX inhibits protein synthesis and therefore memory consolidation and reconsolidation, and extinction memories have been shown to be susceptible to CHX. Thus, we suggest the latter interpretation above is correct: that CHX had an effect on both memories. After 6 minutes of extinction, a sufficient amount of the reactivated fear memory was active, as was the newly acquired extinction memory, making both memories labile to disruption by CHX due to the level of activation.
Results from Experiment 2 confirmed our speculation that both memories were active and susceptible to disruption, in that as the post-extinction injection delay increased the reactivated fear memory consolidated and was then stabilized, allowing the extinction memory to be the dominant, active memory trace. Our results indicate that with a delay of 75 minutes amnesia for extinction is obtained, leaving the fear memory intact. These competing memories, as Eisenberg et al. [25] pointed out, are in behavioral control by being “the one that displays transient sensitivity to the [amnestic treatment]” (p. 1104). The results here suggest that perhaps beyond the cue exposure duration [25, 26] determining which memory becomes the dominant trace, the passage of time following cue exposure may be another important factor as to which memory trace gains behavioral control. Moreover, there appears to be a limited window of time in which the newly formed extinction memory is also susceptible. When the post-extinction delay extended to 120 minutes, retrograde amnesia for extinction was not observed. Thus, with a longer post-extinction injection delay both the reactivated memory and the extinction memory consolidated and were not susceptible to disruption.
It is important to understand when extinction gains control in order to optimally enhance the effects of extinction. Neurobiological models of extinction maintain that extinction is (1) N-methyl-D-aspartate receptor (NMDAr) dependent, (2) impaired when NMDAr antagonists are administered both systemically and centrally, and (3) facilitated by NMDAr agonists [27, 28]. Myers and Davis [28] report that extinction can be blocked when NMDAr antagonists are administered directly or systemically into the basal lateral amygdala (BLA) prior to extinction and directly into the CA1 region of the hippocampus. These findings could apply to humans in a clinical setting since exposure-based therapies aim to extinguish the behavioral responses to phobias, anxiety, and post-traumatic stress disorder (PTSD). By better understanding when an extinction memory gains control, clinicians may one day maximize extinction by administering NMDAr agonist in a time-dependent fashion [see 29, 30].
These present findings also support the view of extinction as new learning and as a separate event from original acquisition [22, 30]. In concordance with the view of extinction as a separate event, the results presented here suggest a temporal gradient for the reconsolidation of a reactivated memory when competing with a newly acquired extinction memory. Although extinction memories have been disrupted using a variety of agents, as well as shown to share similar characteristics with original acquisition memories, there is evidence that the formation of extinction memories is mechanistically different from the formation of original acquisition [31-33]. Moreover, the distinction between reactivation/reconsolidation of an original memory and the consolidation of an extinction memory may depend on cue-exposure duration, the number of cue exposures [33-35], as well as experimental paradigm employed [36]. Since relatively little research has been done on competing memories, there is clearly a need for further research regarding how these two memories interact.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Partial funding of this research was provided by a Susquehanna University Faculty Scholarship Grant to JFB. The authors acknowledge the helpful contributions of ML Klotz and Sara Sheaffer to this project.