All published articles of this journal are available on ScienceDirect.
Social and Behavioral Rhythms is Related to the Perception of Quality of Life in Old Adults
Abstract
Introduction:
The purpose is to verify in old adults if social and behavioral rhythms (SBRs) are correlated with a positive perception of the quality of life (QoL). Social and behavioral rhythms and related circadian biorhythms are known as central points in the pathophysiology of bipolar disorders. A secondary aim is to see if a similar relationship can be found in Major Depressive Disorder (MDD) in old adults. Sample: 141 people aged ≥65 years (58.9% Female).
Methods:
Each subject was evaluated using the Social and Behavioral Rhythms Scale (in which higher scores show more dysfunctional SRBs); SF-12 for QoL and a screening tool for depressive symptoms. They underwent a medical evaluation and blood level assays including cholesterol and triglycerides. The medical diagnoses including MDD were taken into account.
Results:
The Social and Behavioral Rhythms Scale score correlated inversely with SF-12 score (p<0.001) and positively with PHQ9 (p<0.0001). People with MDD had a higher score on social rhythms than controls without (p<0.01). The study highlighted, for the first time, that social and behavioral rhythms have a role in old adults living in the community.
Conclusion:
Further longitudinal studies with a sufficient number of individuals will be required to confirm these data and clarify causal links of the association.
1. INTRODUCTION
Life expectancy has increased worldwide in the last decades and the proportion of older people with a lack of autonomy and disabilities has also increased. This implies significant social and health costs [1]. It is therefore essential that research on the health of older adults must not be limited to the study of pathologies, but also focus on positive well-being and factors that can be used as preventive tools [2, 3].
An element that may affect the quality of life of older adults is the social and behavioral rhythm (SBR) [4]. SBR is connected to external contingencies, one of which was the recent lockdown caused by the Covid-19 pandemic. However, this is also strongly linked to the biological rhymes of the organism. Thus, every change in circadian biological rhythms may influence SBR [4].
The rhythmicity of metabolic and hormonal processes is controlled by a complex endogenous system of pacemakers in which Melatonin [5, 6] and Cortisol [7, 8] play a central role among the mediators. This system affects, but can also be affected by, external factors such as light/dark-cycles, nutrition and exercise and social activities [9]. In humans, melatonin reaches maximum plasma levels at night around 3 to 4 AM. Its release is regulated by a veritable central circadian rhythm generator as the suprachiasmatic nucleus of the anterior hypothalamus [5]. As is known, melatonin regulates body temperature, sleep and promotes sleep onset, and regulates the photosensitive retinal ganglion cells that provide signals to the SCN [5, 6]. Total melatonin secretion declines with age [10, 11]. However, melatonin rhythm is better preserved in healthy older adults [12, 13] in contrast to old people with several neuropathologic illnesses [14-18]. Like melatonin, cortisol rhythms show age-related changes such as a reduction in amplitude with higher secretion at night [7, 8] and delay in the peak in the morning [19].
More alterations of the cortisol rhythm in old humans, as for that of melatonin, were found to be associated with neurodegeneration [20-22]. Organs such as the liver and pancreas are under circadian rhythms in regulating glucose and lipid metabolism [23]. Age-related weakening of circadian rhythms has been hypothesized as exposing older adults [24] to metabolic diseases. Plasma levels of proinflammatory cytokines fluctuate in accordance with rest/activity rhythms [25]. Evidence suggests that dysfunction in circadian clockwork may influence the link between inflammatory response and diet [26] and that age-related changes in rhythms may be related to vulnerability to chronic inflammation and metabolic disorders. Vulnerability to the dysregulation of biological rhythms in older adults has been attributed to reduced responsiveness of the aged master circadian clock to light [27]. Given the increase in light pollution in the modern world and even more so in modern metropolises, this vulnerability exposes the elderly to greater risks [28]. It is also necessary to consider how biological circadian rhythms influence, and may be influenced by, the so-called social and behavioral rhythms.
Older adults show vulnerability to the dysregulation of biological rhythms and, consequently, of SBR. Adults above 60 are significantly more likely to “rising from and retiring to bed earlier than younger adults of 20 and 30 years old” [28]. Indeed, older adults experience changes in the architecture of sleep with more awakenings, longer latencies to falling asleep, and shortening of 3 to 4 rapid eye movement stages while asleep [29] with a loss of around 30 minutes of sleep every 10 years from 40 onwards [30]. These changes, if too accentuated, have been shown to be related cognitive decline and risk of falls [31]. Studies on jet lag, have demonstrated a differential impact on older adults compared with young adults, with poorer sleep efficiency, alertness state, and impaired body temperature [32]. The circadian rhythm of body temperature shows a great decrease in amplitude related to age [33]. The dysregulation of the awakening phases and the consequence of an age-dependent jet lag alter the ability to regulate glucose, and therefore the correct rhythm of nutrition [34], which implies a close relationship between the rhythms of sleep and nutrition. Indeed, evidence has found exercise able to reset disruptions in circadian pacemakers [9] and physical activity is closely linked to social relationships and social activities. Therefore, there is strong evidence that social and behavioral rhythms (such as times of awakening and falling asleep, regularity of food and social relationships), are influenced by, and can influence, circadian biorhythms and therefore may have a relationship with vulnerability to metabolic and inflammatory diseases. Up to now, the alterations of social rhythms and biological rhythms in the elderly have been studied mainly in relation to their vulnerability to pathologies or that greater alteration of the bio and/or social rhythms are directly proportional to the risk of pathologies. This has also been seen in mood disorders in which self-esteem and loss of interest in things is central and whose occurrence is closely related to a poor quality of life. In fact, it has been suggested that there is a possible association between induced irregularity of social and behavioral rhythms (due to fortuitous circumstances and/or to basic irregularity, linked to temperamental / personality profiles), and the risk of mood disorder [35-38]. More specifically, some results indicate that bipolar depression is related to disruption of social and behavioral rhythms in the elderly while unipolar depression is not [39]. However, little evidence is known about the relationship between social rhythm and positive mental health, and perception of life satisfaction, specifically in the elderly.
Our hypothesis is that a good adaptation in the regulation of SBR in the elderly is associated with a better state of well-being and therefore a better quality of life. The main purpose of this work is to verify such hypothesis by administering a randomized controlled trial of physical activity in a sample of elderly people living at home to see if their functional social and behavioral rhythms correlate with a positive health measure such as a good perception of the quality of life (QoL). A secondary purpose is to establish whether the regularity of social rhythms correlates with other parameters related to metabolic rhythms and confirm if there is an association between regularity of social and behavioral rhythms and Major Depressive Disorder in old adults.
2. MATERIALS AND METHODS
2.1. Sample
The study sample included 141 people aged 65 years and older of both genders (83 Females, 58,9%) living at home. They were recruited and preliminarily evaluated for selection to participate in a Randomized Controlled Trial (RCT) on exercise [40]. Inclusion criteria for the preliminary evaluation for eligibility for RCT were having a medical certificate for suitability in non-competitive moderate-intensity physical activity. Exclusion criteria were BMI>35; unsuitability for moderate physical activity due to medical/physical impairment (mild-moderate chronic diseases such as hypertension or diabetes were admitted). This sample had been preliminarily selected and evaluated to exclude the presence of serious diseases by means of a medical examination and was then set for randomization.
2.2. Instruments
Each subject was submitted to a series of instruments as programmed for the RCT design [40]. For the purpose of this study, we took into consideration the measure of Social and Behavioral rhythms (eating, sleeping, social contacts) measured by the Brief Social Rhythm Scale [41].
The Brief Social Rhythm Scale (BSRS) is a simplified tool derived from more complex tools such as the Social Rhythm Metric (SRM) [42], which presented excellent psychometric performances but which were too complex for use in large samples or the context of multidimensional evaluations requiring the use of many tools. BSRS consists of ten items that assess the irregularity with which participants engage in basic daily activities during a week such as the rhythms of sleeping (waking and bedtimes); the regularity in eating (breakfast and dinner) and social contacts with others (at work, school, and leisure time). Participants must rate the regularity of each activity in their lives on a scale ranging from 1 (very regularly) to 6 (very irregularly). Higher scores thus indicate higher irregularity. In this work, the score is considered as a general sum of the individual items. The tool is administered at a single time point. The BSRS has shown good psychometric properties in terms of internal consistency in transnational studies [41]. If the limits for conducting parametric measures for the correlation of the main outcome (BSRS Vs SF-12 score) are met, we will present the linear regression analysis in this measure as a figure with scatterplot.
Health-Related Quality of Life (H-QoL) was measured with the 12-Items Short Form Survey (SF-12) [43]. Depressive Episode and Symptoms were assessed by the Patient Health Questionnaire-9 (PHQ-9) [43]. PHQ9 is a self-administered questionnaire. The PHQ9 score is the sum of the scores of 9 items, each corresponding to one of the key symptoms of the depressive episode DSM criteria. The total PHQ9 score from 0 to 4 indicates minimal symptoms; 5-9 mild minimal symptoms; 10-14, moderate depressive episode; 15-19 moderately severe and 20-27 severe depression [44].
For the diagnoses of MDD, Cancer, Thyroiditis, Diabetes Type 2 and Hypertension, we adopted the criteria reported in the medical assessment adequacy of health conditions for the proposed physical exercise that was part of the trial protocol. We then verified the diagnoses reported by the physical education doctor on the basis of the attached clinical documentation (which, according to the trial protocol, had been requested from each participant). A blinded doctor, therefore, evaluated the PE doctor's diagnosis and the documentation attached to each participant's dossier and confirmed the diagnoses when in accordance with international standards. Thus, for depressive episodes, we have the current status detected by self-assessment (by PHQ9) and the lifetime one for MDD Lifetime Medical diagnosis.
2.3. Ethical Issues
Each participant received detailed explanations about the aims and procedures of the survey. The study was conducted according to the Declaration of Helsinki and its revisions) [45]. The Independent Ethics Committee of the “Azienda Ospedaliera Universitaria di Cagliari” (Cagliari University Hospital), has approved the study (reference number PG/2018/15546 25 October 2018).
2.4. Statistical Methods
For measuring the linear correlation between the score at BSRS, verification of the distribution of normality through the Kolmogorov-Smirnov Test of Normality for each parameter considered for correlation was carried out. The correlation between SBRS total score and each parameter considered was conducted by means of both the Pearson and Spearman Correlation Tests, with the intention of using the latter if the data (for each specific parameter involved in the correlation) did not respect the normal distribution. The comparison between subgroups of participants receiving or not receiving a diagnosis (Thyroiditis, Cancer, Diabetes Type 2, Hypertension, Major Depressive Disorder; Depressive Episode) was carried out for each sub-group against a control sample without the same condition selected by randomization after blocks from the overall sample of people without such a condition. For this purpose, for each person with a condition (for example Thyroiditis), a cell was created with all the people without such a condition and of the same age (+/- 2 years old) and gender, thus two people (but one for Hypertension, 4 for PHQ≥14, MDD in remission without treatment and MDD with antidepressant) were selected by randomization from each cell obtained. The comparison between groups with conditions and matched groups without conditions was carried out by means of the Mann-Whitney Test U (Tables 1 and 2).
| Condition /Measure |
Pearson R P (2 tailed) |
Spearman Rho P (2 tailed) |
Kolmogorov-Smirnov Test of Normality K-S Test Statistic (D) |
|---|---|---|---|
| SBRS | --- | ---- | 0.1045 P=0.081* |
| H-Qol/SF12 | -0.360 p<0.0001 |
-0.238 p=0.0044 |
0.1393 P=0.007** |
| Physical QoL/MSF-12 | -0.154 p=0.0601 |
-0.120 p=0.1542 |
0.1153 P=0.041** |
| Psychological QoL/PSF-12 | -0.262 P=0.0023 |
-0.262 p=0.0017 |
0.1750 P<0.001** |
| Depressive Symptoms/PHQ9 score | 0.396 P<0.0001 |
0.309 P<0.0001 |
0.02259 P<0.001** |
| Total Cholesterol | 0.158 P=0.0602 |
0.157 p=0.06 |
0.06184 P=0.6351* |
| Triglycerides | 0.256 P<0.001 |
0.262 p=0.00165 |
0.13271 P=0.0013** |
| BMI | -0008 P=0.9294 |
-0.007 p=0.930 |
0.21715 P<0.001** |
| Condition |
BSRS Mean±SD In People with the Condition as Described in First Column N=Sample Size F=Number of Females >75Years = Number of People Older than 75 |
BSRS Mean±SD In People without the Condition as Described in First Column N=Sample Size F=Number of Females >75Years = Number of People Older than 75 |
Z | P |
|---|---|---|---|---|
| Thyroiditis | 24.7±8.7 (N=25) [Female=11 >75Years=5] |
19.10±5.2 (N=50) Female=22 >75Y=10] |
-2.860 | <0.01 |
| Cancer | 22.6±9.7 (N=15) [Female=11 >75Years=5] |
22.7±5.7 (N=30 Female=22 >75Years=10] |
0.602 | 0.548 |
| Diabetes Type 2 | 18.0±5.8 (N=12) [Female=4 >75Years=4] |
19.7±7.1 (N=24) [Female=4 >75Years=4] |
0.922 | 0.357 |
| Hypertension | 20.5±3.9 (N=55) [Female=27 >75Years=15] |
21.0±9.9 (N=55) Female=54 >75Years=30] |
0.648 | 0.515 |
| Major Depressive Disorder Lifetime (medical diagnosis) |
24.8±11.9 (N=20) [Female=15 >75Years=5] |
20.8±9.5 (N=40) [Female=30 >75Years=10] |
-2.773 | 0.0056 |
| Depressive Episode PHQ≥10 (55.5% with medical diagnosis of MDD) |
29.3±12.5 (N=9) [Female=8 >75Years=3] |
21.0±8.3 (N=36) Female=32 >75Years=12] |
-4.532 | 0<.001 |
| Previous MDD in remission without treatment | 18.5±7.2 (N=8) [Female=6 >75Years=2] |
20.7±9.2 (N=32) [Female=24 >75Years=8] |
0.311 | 0.756 |
| Chronic Depression with antidepressants (medical diagnosis) | 28.4±13.0 (N=8) [Female=7 >75Years=1] |
20.3±8.3 (N=24) Female=28 >75Years=4] |
-6.813 | 0.010 |
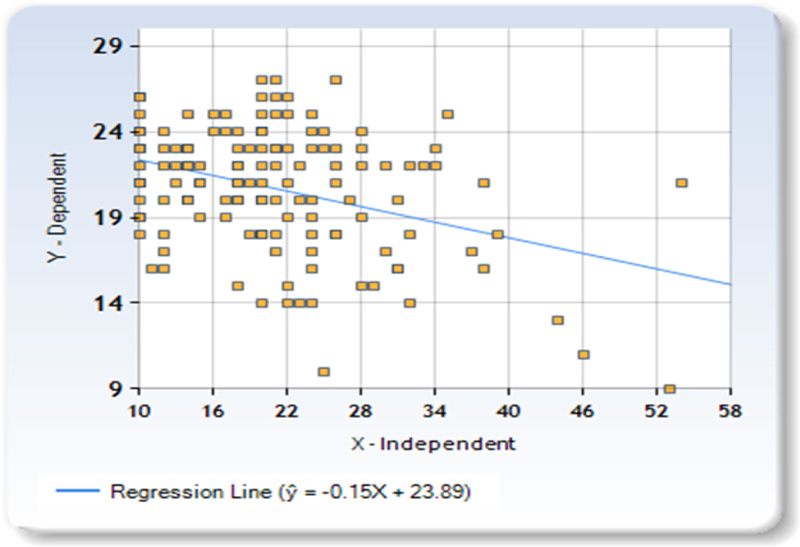
Sum of X (BSRS SCore) = 2938Sum of Y (SF-12 score) = 2922 Mean X = 20.8369 Mean Y = 20.7234 Sum of squares (SSX) = 10533.2482 Sum of products (SP) = -1598.3617BRegression Equation = ŷ = bX + aBb = SP/SSX = -1598.36/10533.25 = -0.15174Ba = MY-bMX = 20.72 - (-0.15*20.84) = 23.88528 Bŷ = -0.15174X + 23.88528.
3. RESULTS
The distribution of SBRS score in the sample respected the normal distribution according to the Kolmogorov-Smirnov Test of Normality and the same was true for the level of cholesterol in the blood. For all the other measures examined it did not; we, therefore, referred to the results of Spearman's non-parametric test. However, the results of the two tests (the parametric Pearson and the non-Parametric Spearman) were superimposable.
The score at SBRS of each participant was shown in an inverse linear correlation between the score at SF-12 (measuring H-QoL) of the same individual, which means that the higher the score at BSRS (i.e., more dysfunctional social rhythms) the lower the score at SF-12 (i.e., more worsened was H-QoL) (Rho=-0.238, p<0.01). The result was confirmed when comparing the SBRS score with the component of the SF-12 that measures psychological well-being (Rho=-0.262, p<0.01) while no inverse relationship between BSRS and SF-12 for quality of physical life was shown (Rho=0.1542; p=0.120). On the contrary, the score for SBRS was in a linear correlation with the score of PHQ9 which measures Depressive Symptoms (Rho=0.309; p<0.001). The SBRS score correlated positively with blood triglyceride dosage (Rho=0.157, p=0.06) but did not with blood cholesterol dosage (Rho=0.157, p=0.06) or with BMI (Rho=-0.007, p=0.930). The subgroups of people who had cancer or type 2 diabetes or hypertension did not have a higher mean SBRS score than those who did not have the same disorder, people suffering from thyroiditis did have a higher score (24.7±8.7 vs. 19.10±5.2, Z=-2.860p<0.01.)
A Lifetime Major Depressive Disorder medical diagnosis was associated with a higher score at the scale of social rhythms (24.8±11.9 vs 20.8±9.5, Z=-2.773, p=0.0056) as well people with a score at PHQ9≥10 (identifying a current depressive episode) (29.3±12.5 vs 21.0±8.3, Z=-4.532, P<0.001) and those with Chronic Depression (medical diagnosis of Lifetime MDD) taking antidepressants (28.4±13.0 vs 20.3± 8.3, Z=-6.813, P=0.010). People with previous MDD in remission (i.e. with lifetime medical diagnosis of MDD but negative at PHQ9) and currently without treatment showed no difference in social or behavioral rhythms with respect to their respective control group. Since the limits for conducting parametric measures for the correlation of the main outcome (BSRS Vs SF-12) score were met, we then presented the linear regression analysis of this measure in Fig. (1) with a scatterplot.
4. DISCUSSION
To our knowledge, our study is the first to show that in a sample of elderly adults, social and behavioral rhythms are related to positive well-being, i.e. having a functional and stable rhythm as measured by the SRBS scale (in which lower scores indicate more functional rhythms) is inversely related to the SF-12 score which measures the quality of life, with a higher score indicating a higher perceived QoL.
Such a result (relationship between social rhythms and quality of life) had instead been found in numerous studies on mood disorders. Especially studied was the link between sleep and quality of life in Bipolar Disorders [37, 38]. Data on Major Depressive Disorder were conflicting [39]. Our study shows that the SRBS score correlates positively with the PH9 score, which is an indicator of depressive symptomatology at point prevalence. PHQ9 shows high scores during a depressive episode. A depressive episode (with a high PHQ9 score) can occur in both Major Depressive Disorder and Bipolar Disorder (these are lifetime diagnoses that both involve the presence of depressive episodes), claiming that the correlation between SRBS/PHQ9 indicates a link between the presence of Major Depressive Depression and the high score at SRBS may be biased by the presence of people with Bipolar Disorders. However, Bipolar Disorder has a lower frequency in the community and, what is more, in older people, than does Major Depressive Disorder, thus the weight of Major Depressive Disorders in the correlation is higher than that of Bipolar Disorder.
However, the association between Major Depressive Disorder and Rhythms is also confirmed by the higher scores on the SRBR scale among all those who have had a specific medical lifetime diagnosis of Major Depressive Disorder compared to those who have not. In conclusion, the results indicate that in the elderly social and behavioral rhythms are strongly altered in Major Depressive Disorder in older adults. The presence of a dysfunctional profile of social and behavioral rhythms is confirmed in those with chronic depression while suffering from a depressive episode in the past is not associated with altered rhythms in people who had recovered from it (i.e. people with MDD diagnosis but negative at PHQ at current evaluation). This element is relevant since it suggests a possible role in improving circadian rhythms in a recovery path as well as a possible role in prevention, although a cross-sectional study cannot explain the causal sense of this association (i.e. if it is the healing of depression that causes the return to a functional rhythm or if a factor of recovery is the return to a functional rhythm). This aspect will need to be clarified by future longitudinal studies.
Returning to the main result of the study, which is the demonstrated relationship between social rhythms and quality of life in old adults, it is necessary to underline that our results concern only the correlation of the overall score at SBRS with the measure of the quality of life. This is an important result but very difficult to interpret. It is therefore a preliminary picture that suggests the need for future research to better understand the details of the interaction of the specific components (sleep, eating, having social contacts) and to clarify the role of the biological clocks of rhythm. Although we made it clear in the introduction that all aspects of social rhythms are interconnected, by far the aspect most studied so far has been the sleep-wake rhythm as well as the biological markers of this rhythm. For example, incorrect eating habits, such as irregularity in dietary rhythms, may conceivably be related to the kind of food intake, and thus may have consequences on lipid metabolism.
It is well known that dysregulation of light-shadow rhythms can directly influence sex hormones as the block of melatonin at night, due to loss of sleep and excess of light, may influence estradiol/progesterone balance in favor of estradiol [46]. Exposition to light pollution can in fact induce the risk of breast cancer and prostate cancer [47] as well as mood disorders [28]. But it is also known that circadian modifications of melatonin can cause changes in the metabolism of lipids [48]. The consequences of metabolic dysfunctions and obesity of the de-synchronization of biological rhythms due to the effect of artificial light have been hypothesized [49] and are currently studied particularly by Arab researchers concerning the coping mechanism in Ramadan [50]. However, the impact of light disruption on obesity has not yet been clarified and this study, despite having highlighted a relationship between social and behavioral rhythms and blood lipid parameters, has revealed no relationship with BMI.
The blocking of melatonin can affect insulin resistance and carbohydrate metabolism [51] and it has recently been reported that exposure to excess light may induce metabolic syndromes [52]. In our study, however, no direct relationship between altered social rhythms and type II diabetes and BMI was found. But the study did highlight that having dysfunctional rhythms can be related to lipid metabolism because it has been seen that the scores on the SRBS correlate inversely with the plasma levels of triglycerides. This suggests an influence of social rhythms on some (but not all) aspects related to metabolism, but the sample was not large enough to encompass an analysis of specific aspects of social rhythms that could possibly affect everyone as previously underlined.
From this point of view, it is interesting that our study has verified a close relationship between the alteration of social patterns (which are closely related to biological rhythms) and thyroiditis. Even in this case, however, the causal link of the association needs to be clarified. Thyroiditis is a chronic disorder and hormonal dysfunction may alter the control of food and sleep. Longitudinal studies with adequate power and sample size will therefore be needed to investigate this link.
The study found no relationship between altered social rhythms and cancer or hypertension. In the first case, it must be remembered that this is a sample of people who, although they may have medium-mild severity disorders, must have tested positive for a medical examination for sports activities. Therefore, they were people in healthy condition who in the past and at present had overcome a neoplastic pathology. It is reasonable to assume in this case that the regulation of biological rhythms can even accentuate the improvement. In the same way, it is possible to hypothesize that a self-regulation mechanism may also have occurred in arterial hypertension.
CONCLUSION
The study highlights, for the first time, that the rhythm of social relationships and habits such as sleep and nutrition have a role in the well-being of old adults. The dysregulation of individual rhythms was found related to disorders such as depression, but also biological parameters related to dysmetabolism. Further longitudinal studies with a sufficient number of individuals will be needed to confirm these data and clarify the causal link of the association between QoL and SBR.
LIST OF ABBREVIATIONS
| QoL | = Quality of Life |
| MDD | = Major Depressive Disorder |
| SBR | = Social And Behavioral Rhythm |
| H-QoL | = Health-Related Quality of Life |
| BMI | = Body Mass Index |
| RCT | = Randomized Controlled Trial |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical committee of the “Azienda Ospedaliero-Universitaria di Cagliari” approved the study on October 23rd, 2018 (reference number PG/2018/15546).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Each participant signed informed consent.
STANDARD OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The “Active Aging Study” Identifier: NCT03858114. Clinical Trial.Gov was funded by a grant from “Fondazione di Sardegna”.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENETS
Declared none.


